Fig. 46.1 Anterior posterior chest x-ray showing a cardiac device
■
A 65-year-old female after a motor vehicle collision requires emergency surgery for an open lower extremity fracture; the patient tells you she has a “bad heart,” she has no history in your institution, and no signs of heart failure. An EKG shows wide QRS with dual-chamber pacing. A CXR on admission show (See Fig. 46.1).
- 1.
What type of device is shown in the image?
- 2.
What are the indications for cardiac implantable electronic device placement?
- 3.
What is the effect of placing a magnet over the device (pacemaker and/or ICD)?
- 4.
In the OR, you place a magnet over the device. The patient goes pulseless after prolonged use of electrocautery. What is your diagnosis?
- 5.
What are the effects of electrocautery, radiation therapy, and radiofrequency on a pacemaker and an ICD?
- 6.
What measures can you take to ensure proper intraoperative device functioning?
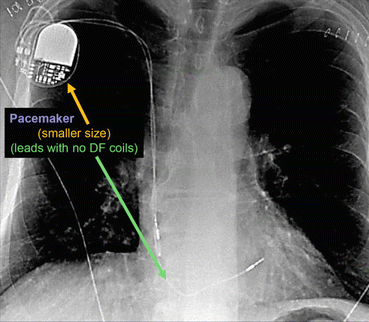
Fig. 46.2 Anterior posterior chest x-ray showing a pacemaker
- (a)
The radiographic image of a pacemaker would show (See Fig. 46.2):
Smaller generator
Discreet right ventricular lead (stable diameter)
With or without right atrial lead or coronary sinus lead
- (a)
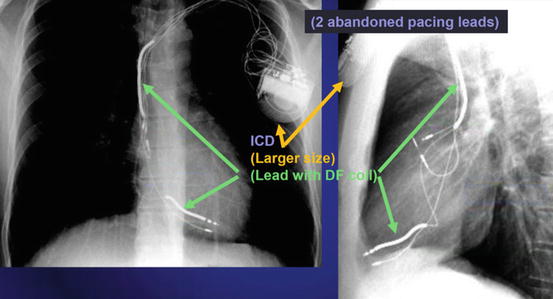
Fig. 46.3 Anterior posterior and lateral chest x-ray showing an ICD
- (b)
The radiographic image of an ICD would show (See Fig. 46.3):
Larger generator.
Prominent right ventricular lead, otherwise known as shock coils. They appear as two metallic segments along the length of the ICD lead.
- (b)
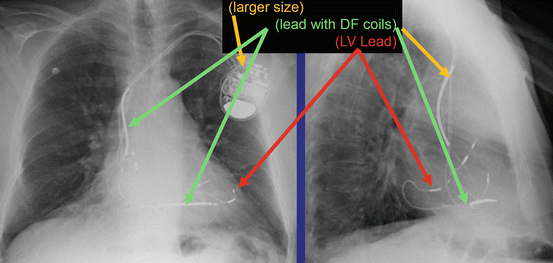
Fig. 46.4 Anterior posterior and lateral chest x-ray showing a BiV ICD
- (c)
The radiographic image of a BiV ICD would show (See Fig. 46.4):
Larger generator
Prominent right ventricular lead (shock coils)
Right atrium lead
Coronary sinus lead
- (c)
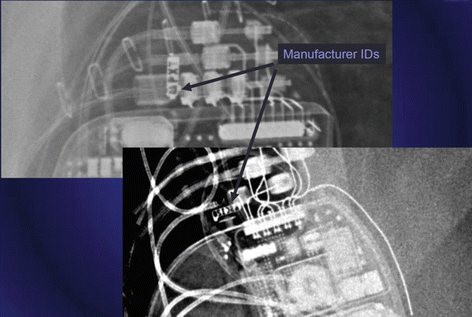
Fig. 46.5 Magnified view of a chest x-ray showing manufacturer ID of a cardiac implantable electronic device
Manufacturer ID can be seen in the CXR as well (See Fig. 46.5).
- 2.
Indications for cardiac implantable electronic device placement [2]:
- (a)
Pacemaker:
Patients with symptomatic sinus node dysfunction and bradycardia
Patients with complete AV block (symptoms less relevant)
Hypersensitive carotid sinus syndrome and neurocardiogenic syncope
- (b)
ICD:
Patients at risk of sudden cardiac death: Prior ventricular tachycardia or fibrillation, low ejection fraction [3]
Long QT syndrome
Hypertrophic cardiomyopathy
Arrhythmogenic right ventricular dysplasia
Cardiac transplantation
Primary electrical disease: idiopathic ventricular fibrillation, short QT syndrome, Brugada syndrome, and catecholaminergic polymorphic ventricular tachycardia
- (c)
BiV ICD:
Treatment of left ventricular dysfunction and heart failure, with prolonged ventricular conduction and heart failure symptoms.
Required ventricular pacing and low EF:
RV pacing in patients with low EF increases CHF admissions and mortality.
Cardiac resynchronization therapy [4]:
Improved exercise tolerance and mortality.
Continuous pacing provides better hemodynamic stability.
- (a)
- 3.
Effect of a magnet on a device [5]:
Get Clinical Tree app for offline access
- (a)
Pacemaker:
Suspend sensing of intrinsic rhythm.
Pacing in an asynchronous mode: the rate depends on the manufacturer and the battery life; if the battery life is low, the rate may not be adequate for surgery.
Turns off “rate response.”
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



- (a)

