3 Nguyen Do and Dan Miulli The overall incidence of olfactory nerve dysfunction is 7%, increasing to 30% with severe head injuries or anterior cranial fossa fractures. Injury is difficult to establish in an acute trauma phase where nasal bleed and swelling confound the examination. In ~50% of cases, anosmia is only of temporary duration. The reason for the return of olfaction is not entirely clear. Recovery of the sense of smell can be expected at any time from a few days up to 5 years.1 The primary feature is decreased or absent sense of smell. Two objective tests of olfaction are olfactory respiratory reflex and olfactory electroencephalography (EEG). The former helps in ruling out malingering, and the latter provides a nonspecific α response to an odoriferous substance. The olfactory respiratory reflex is performed with the subject seated and blindfolded. This test is based on the principle that sudden inhalation of an odor will cause a temporary arrest in the normal respiratory rhythm. This is a fairly good test to detect malingering. The olfactory EEG is performed with the eyes closed. Once the α rhythm is well established, which may take some time, an odoriferous substance is introduced. If olfaction is present, the α rhythm is abolished. This is a nonspecific response but is of value in identifying malingerers.2 A high-resolution computed tomography (CT) scan of the ethmoids and frontal fossa is essential in the workup of anosmia, especially with cerebral spinal fluid (CSF) rhinorrhea, which frequently accompanies trauma to the anterior cranial fossa and nasal sinuses. Magnetic resonance imaging (MRI) of the brain can be useful in showing injury to the orbital surface of the frontal lobe, often observed in association with injury to the olfactory bulbs.3 Because there is no specific treatment, patients with post-traumatic anosmia can only be counseled with the known statistical information regarding their potential for recovery.4 Recovery occurs in up to 50% of cases, usually during the first 3 months, but it may be up to 5 years after injury.2 Trauma to the orbit, with or without significant craniocerebral trauma, is rarely neatly circumscribed, and a severe injury to the eye may involve varying admixtures of optic nerve, extraocular muscle and nerve, and optic globe insults.1 The optic nerve is tethered in the optic canal and is subject to stretch, causing injury during brain shifts. Injury to this part of the optic nerve occurs in association with direct trauma to the globe as the nerve is pushed posteriorly and suffers a partial or complete avulsion at the back end of the globe. The ophthalmoscopic picture consists of a marginal hemorrhage extending to the disk. On visual field examination, there is a sector defect extending from the blind spot to the periphery.5 Although fractures of the orbit are common, isolated injury to the intraorbital portion of the optic nerve is rare. With severe trauma to the apex of the orbit, there can be a disruption of the sphenoid fissure with loss of function of the third, fourth, and sixth nerves, and the ophthalmic branch of the fifth nerve, accompanied by monocular blindness and proptosis secondary to hemorrhage into the muscle cone.5 The most vulnerable component of the optic nerve is that portion of the nerve located within the optic canal. Although the intracanalicular portion of the nerve may be injured directly by penetrating foreign objects, the majority of cases follow closed head injuries, primarily those involving frontal, temporal, and orbital trauma.5 The chief clinical feature is decreased or loss of vision. Scotomas, sector, and altitudinal defects can occur. Most traumatic optic neuropathies result from severe head trauma, and altered consciousness in the patient delays the diagnosis. An afferent pupillary defect in an unconscious patient is useful in the detection of the optic nerve injury, whereas in conscious patients, testing for visual fields and acuity usually establishes the diagnosis.3 With a complete injury to the optic nerve within the canal, there is monocular blindness, a dilated pupil with an absent direct pupillary response, and a brisk consensual response to light. The funduscopic appearance is unremarkable initially, but atrophy of the disk develops in several weeks. The pupil on the affected side is larger than the uninvolved one, and there is some diminution in the pupillomotor response to consensual stimulation.6 CT scan and/or MRI is useful to assess for possible basal skull fractures or compressive lesions. Clinical evaluation and investigations such as electroretinography and visual evoked potentials must be combined when evaluating visual impairment.4 Treatment is controversial for indirect optic neuropathy and includes observation, high-dose steroids, and surgery. In patients with delayed-onset loss of vision from compression of the optic nerve and failed steroid treatment, operative approaches for optic nerve decompression include transcranial, transethmoidal, transmaxillary, and transorbital routes.5 The prognosis for restoration of vision in a patient with an optic nerve injury is poor. Forty to 50% of patients remained blind, and up to 75% showed no improvement in their reduced visual acuity or in a documented field defect. If improvement is to take place spontaneously, it does so within the first several days and continues for 4 to 6 weeks, at which time the condition becomes stationary. The prognosis is said to be better in those patients whose visual acuity is diminished but who retain a good pupillary response to light.3 The oculomotor nerve projects from the anterior part of the midbrain to the tentorial incisura at the level of the posterior clinoid processes in an open V-shaped fashion. The third nerve probably becomes damaged by a frontal blow to the accelerating head that results in stretching and contusion of the nerve. The most common site of trauma is believed to occur at the point where the nerve enters the dura mater at the posterior end of the cavernous sinus. Bilateral third nerve injuries are extremely uncommon.7 There is paralysis of medial rectus, superior rectus, inferior rectus, inferior oblique, levator palpebrae superior, and constrictor ciliary muscles.3 Clinical features include ptosis, outward eye deviation, dilated pupil, and no reaction to light or accommodation. The diagnosis of oculomotor nerve injury in conscious and cooperative persons is not difficult. In unconscious patients, especially those with orbital bruising and hematoma, a good history with regard to previous oculomotor status and the findings of the immediate post-traumatic examination are of great help in making an early diagnosis.7 Diplopia fields, CT, and/or MRI scans should be used to look for a compressive lesion. The treatment is initially symptomatic and consists of wearing a patch over the eye to prevent troublesome diplopia. If recovery does not occur in 4 to 6 months, then local muscle shortening procedures can be performed in the affected eye in certain situations.7 Recovery begins within 2 to 3 months when the nerve is in continuity. Aberrant regeneration often occurs with findings such as lid elevation and pupillary constriction with attempted adduction or depression.3 The fourth cranial nerve is the least frequently injured ocular motor nerve. When involved, the nerve is damaged by contusion or stretching as it exits the dorsal midbrain near the anterior medullary velum. In severe frontal blows against the accelerating head, the midbrain is displaced against the posterolateral edge of the tentorial incisura, causing contusion, hemorrhage, and damage to one or both fourth nerves.1 There is paralysis of the superior oblique muscle resulting in the impaired ability to turn the eye down and in. Injury to the trochlear nerve is rarely diagnosed. Often a complaint of diplopia after recovery from a severe head injury leads to this diagnosis. Typically, the double vision is vertical, especially when the patient walks down a flight of stairs.3 CT and/or MRI scans should be used to look for a compressive lesion. Treatment is symptomatic, such as an eye patch, semitransparent tape to glasses, and prisms for diplopia. Muscle-shortening procedures may be helpful for permanent diplopia.4 Only 50% of patients recover because of frequent avulsion of the trochlear nerve in the traumatic process.1 The abducens nerve is injured, along with the seventh and eighth cranial nerves, in fractures of the petrous bone. Vertical movement of the brainstem during trauma can severely stretch or avulse the sixth nerve as it leaves the pons before it enters the clival dura.8 Increased intracranial pressure or herniation can cause delayed secondary paralysis of the cranial nerve (CN) VI. The abducens nerve can also be injured along with CN III and IV at the superior orbital fissure.9 There is paralysis of the lateral rectus muscle, resulting in inward eye deviation. Diplopia occurs in all gaze directions. Lateral rectus palsy in its minor form may not correlate well with abducens nerve injury, and conjugate movements that are controlled by the brainstem sometimes make the diagnosis of abducens nerve palsy difficult to establish.1 The diagnosis of abducens palsy in the unconscious patient can be made when the affected eye fails to wander outward spontaneously, abduct when the head is passively turned away from the side of the sixth nerve paralysis, and abduct in response to ipsilateral cold caloric irrigation.3 CT and/or MRI scans should be used to look for a compressive lesion or underlying fractures. The treatment is initially symptomatic and consists of wearing a patch over the eye to prevent troublesome diplopia. If recovery does not occur in 4 to 6 months, then local muscle-shortening procedures can be performed in the affected eye in certain situations. Botulinum toxin, injected into the ipsilateral medial rectus muscle, has also been proposed as a treatment option for a faster recovery.8 Many cases of abducens palsy recover spontaneously after ~4 months, a period of time consistent with axonal regeneration.4 Lesions affecting the medial longitudinal fasciculus (MLF) cause failure of the adducting eye to move, whereas the abducting eye deviates laterally to its full extent but has accompanying nystagmus during conjugate deviation. The medial rectus adducts without difficulty during convergence, distinguishing the paresis from a CN III or muscle lesion. This pattern of disconjugate eye movements is referred to as internuclear ophthalmoplegia (INO) because the lesion disconnects the nuclei of cranial nerves III and VI by causing failure of neural conduction in the internuclear pathway, the MLF.4 The monocular nystagmus can be either transitory (1 or 2 beats) or sustained. When the degree of dysfunction in the MLF is mild, the adducing eye may deviate fully but cannot attain the proper velocity during a saccadic refixation. In this case, there is a noticeable dissociation between the two eyes during the saccade, because the abducting eye completes the movement earlier than the adducting eye.1 The clinical importance of diagnosing the MLF syndrome is its localizing value for lesions deep in the substance of the brainstem tegmentum. This general area in the brainstem contains the ascending reticular activating system, which is necessary for consciousness, along with several adjacent cranial nerve nuclei, and various ascending and descending sensory and cerebellar pathways.4 Therefore, the isolated occurrence of an MLF syndrome in an alert individual without other brainstem signs or symptoms suggests the presence of a highly discrete lesion. This is caused by small demyelinating plaques of multiple sclerosis or by small infarctions due to small vessel disease; very occasionally it can be encountered in the setting of head trauma.3 Trigeminal nerves and branches are commonly injured with facial trauma, especially supraorbital and supratrochlear nerves as they emerge from the supraorbital notch and superomedial aspect of the bony orbit. The infraorbital nerve is often injured in orbital floor blow-out fractures.8 There is paralysis of muscles of mastication with deviation of the jaw to the side of the lesion; loss of sensation for touch, temperature, or pain in the face; and loss of corneal reflexes.4 CT and/or MRI scans should be used to exclude underlying fractures. Treatment consists of decompression of the infraorbital nerve in orbital floor fractures. However, symptomatic relief of hyperpathia due to supra- and infraorbital neuropathies can be accomplished with medications such as carbamazepine, baclofen, pimozide, phenytoin, capsaicin, clonazepam, and amitriptyline. Carbamazepine is most commonly used with complete or acceptable relief achieved in up to 69% of cases. Baclofen may be more effective as an adjunct to carbamazepine.8 Surgical options include peripheral nerve blocks by phenol or alcohol or neurectomy, percutaneous rhizotomy, Spiller-Frazier retrogasserian rhizotomy, microvascular decompression, and stereotactic radiosurgery. Percutaneous trigeminal rhizotomy is preferred in patients who are at high risk for major surgery and low life expectancy (<5 years). Rhizotomy is performed using radiofrequency or balloon compression, and results are comparable. Microvascular decompression provides long-lasting relief and a low incidence of facial anesthesia. Up to 90% of recurrences are in the distribution of the previously involved nerve divisions; 10% involve a new one.1 Hyperpathia in the distribution of the nerve may be permanent. Trauma is the second most common cause of facial paralysis after Bell’s palsy. The long, tortuous, intraosseous course of the facial nerve in the temporal bone makes it highly susceptible to injury in temporal bone fractures. In ~50% of cases of transverse temporal bone fractures, the facial nerve within the internal auditory canal is damaged. With longitudinal fractures, the nerve is not directly involved, but a delayed paralysis may ensue secondary to edema.10 Trauma involving the internal auditory canal injures both facial and vestibulocochlear nerves, resulting in facial nerve symptoms, loss of hearing, and vertigo. Lacerating injuries of the face can cause total or partial paralysis of the face depending on the branches involved.1 There is paralysis of facial muscles with or without loss of taste on the anterior two thirds of the tongue or altered secretion of the lacrimal and salivary glands (nervus intermedius). Lesions near the origin of the nerve or near the geniculate ganglion lead to loss of motor, gustatory, and autonomic functions. If lesions occur between the geniculate ganglion and the origin of the chorda tympani, lacrimal secretion will not be affected. If the trauma occurs near the stylomastoid foramen, there will only be facial paralysis.4 A systematic clinical examination of the facial nerve and its branches can be performed to pinpoint the location of damage to the nerve in the fallopian canal. The testing should include tear production by the Schirmer test (greater superficial petrosal nerve), saliva secretion, taste in the anterior two thirds of the tongue (chorda tympani), and the reflex reaction of the stapedius muscle.1 Electrodiagnostic studies of the injured facial nerve should be done promptly in every case to serve as a baseline for subsequent follow-up examinations. Electromyography (EMG) of the face, the transcutaneous nerve excitability test (NET), and evoked EMG are the most favored methods at present. Radiologic studies should include high-resolution CT scanning with appropriate bone windows to show the fracture in greatest detail.11 Monitoring is the preferred treatment, because an excellent spontaneous recovery can be expected with delayed-onset paralysis. With no surgical management, 90% of patients experience good recovery within 6 months. The peripheral supportive devices in the form of hooks and dental bars to support the sagging face do not achieve their goal and are not recommended, especially in the waiting period. Reassurance regarding the chances of recovery and active exercises in front of a mirror, in case of partial paralysis, are helpful. Artificial tears and an eye patch at night are mandatory to prevent exposure keratitis.4 Absent facial nerve stimulation after 4 days may indicate surgical explorations, especially with transverse fractures of the temporal bone and a discontinuous fallopian canal. The surgical methods used include decompression of the nerve and nerve suturing, either directly or with a cable graft to bridge the nerve defect. In the delayed type of facial paralysis, only decompression is required. In immediate types of facial nerve injury when the nerve is found to be transected, meticulous micro-suturing should be performed to approximate the nerve fascicles.3 Various grafting techniques have also been used with good results. When the nerve is transected outside the stylomastoid foramen, grafting has been done with the sural or great auricular nerves. Hypoglossal-facial anastomosis and a sural nerve graft from the premeatal facial nerve stump to the facial nerve distal to the stylomastoid foramen are other available methods. Plastic surgical procedures on the face in the form of slings and facelift operations can be performed in selected cases when the facial paralysis has been determined to be permanent or when neural repair is not feasible.1 Spontaneous recovery is usual after longitudinal fractures. When due to transverse fractures, 50% recovery after decompression has been found (Table 3–1).11
Cranial Nerve Injuries and Their Management
 Olfactory Nerve Injuries
Olfactory Nerve Injuries
Site of Trauma
Clinical Features
Evaluation
Management
Prognosis
 Optic Nerve Injuries
Optic Nerve Injuries
Site of Trauma
Trauma to the Intrabulbar Portion of the Optic Nerve
Trauma to the Intraorbital Portion of the Optic Nerve
Trauma to the Intracanalicular Portion of the Optic Nerve
Clinical Features
Evaluation
Management
Prognosis
 Oculomotor Nerve Injuries
Oculomotor Nerve Injuries
Site of Trauma
Clinical Features
Evaluation
Management
Prognosis
 Trochlear Nerve Injuries
Trochlear Nerve Injuries
Site of Trauma
Clinical Features
Evaluation
Management
Prognosis
 Abducens Nerve Injuries
Abducens Nerve Injuries
Site of Trauma
Clinical Features
Evaluation
Management
Prognosis
 Internuclear Ophthalmoplegia and Medial Longitudinal Fasciculus Syndromes
Internuclear Ophthalmoplegia and Medial Longitudinal Fasciculus Syndromes
 Trigeminal Nerve Injuries
Trigeminal Nerve Injuries
Site of Trauma
Clinical Features
Evaluation
Management
Prognosis
 Facial Nerve Injuries
Facial Nerve Injuries
Site of Trauma
Clinical Features
Evaluation
Management
Prognosis
| Grade | Descriptor | Detailed description |
| 1 | Normal | Normal facial function in all areas |
| 2 | Mild dysfunction |
|
| 3 | Moderate dysfunction |
|
| 4 | Moderate to severe dysfunction |
|
| 5 | Severe dysfunction |
|
| 6 | Total paralysis | No movement |
 Vestibulocochlear Nerve Injuries
Vestibulocochlear Nerve Injuries
Site of Trauma
Hearing and labyrinthine dysfunction occur in head injuries due to damage to the auditory or vestibular nerves and their end organs, or trauma to the middle ear and conducting elements such as the ossicular chain. Conductive hearing loss follows longitudinal temporal bone fractures in over 50% of cases. Transverse fractures result in vestibular and cochlear nerve laceration in over 80% of cases. In transverse fractures of the petrous bone, the anterior portion of the vestibule and the basilar turn of the cochlea are often damaged, and many of these patients will have an accompanying facial paralysis. Patients with a fracture involving the optic capsule will often develop total degeneration of the cochlear and vestibular end organs.1
Clinical Features
Findings associated with a fracture of the petrous portion of the temporal bone include hemotympanum or tympanic membrane perforation with blood in the external canal, hearing loss, vestibular dysfunction, peripheral facial nerve palsy, CSF otorrhea, and ecchymosis of the scalp over the mastoid bone (Battle’s sign). Benign positional vertigo occurs in ~25% of patients following head trauma. Vestibular symptoms are often not evaluated with caloric stimulation in the acute phase for fear of introducing infection in the presence of a perforated drum. These tests should be done once the patient is stable and any perforation of the tympanic membrane has healed if vestibular symptoms still persist. In a conscious individual, the absence of nystagmus on caloric testing indicates damage to the vestibular nerve or end organ.4 Tinnitus has been reported in 30 to 70% of patients with head injuries. Hearing loss of some degree after head trauma has been reported in over one half of patients with serious head injuries. In the majority of cases, hearing loss is of a sensorineural type, with the conductive variety accounting for only ~3% of cases.3
Evaluation
To evaluate injury, the external canals and tympanic membranes must be examined. An audiogram including pure-tone and speech audiometry, brainstem auditory evoked potential, acoustic reflexes, and middle ear function is used to assess hearing loss. The main radiologic investigation consists of high-resolution CT of the posterior fossa with appropriate window levels to visualize the bony anatomy of the petrous bone. Labyrinthine function is best assessed by caloric stimulation and electronys-tagmography.3
Management
Conductive hearing loss due to ossicular chain disruption can be treated surgically by correcting the middle ear, and it has a good prognosis. There is no specific treatment for sensorineural deafness. A hearing aid may help. Some improvement ensues with partial injuries. Cochlear implants are undergoing clinical trials and hold promise in the treatment of sensorineural deafness.3 Labyrinthine sedatives, such as prochlorperazine, along with avoidance of sudden change of posture and firm reassurance, are the recommended treatment of the minor types of dizziness, vertigo, and nausea that are seen so frequently after trivial head injuries. Positioning maneuvers, such as Epley’s, Hallpike’s, or Semont’s, which move the debris out of the semicircular canal and into the utricle, can be curative for benign positional vertigo.4 Perilymph fistula with loss of eighth nerve function indicates surgical exploration. In refractory post-traumatic vertigo, a labyrinthectomy or translabyrinthine vestibulocochlear nerve section or selective vestibular nerve section (in cases with preserved hearing) may provide relief.1
Prognosis
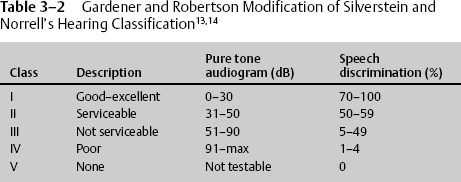
The prognosis for impaired hearing of the conductive type due to hemotympanum or ossicular chain disruption is usually good. Hemotympanum usually resolves spontaneously, and ossicular chain disruption can be corrected surgically in most cases. The prognosis of sensorineural deafness is poor. Some improvement may occur with partial lesions. Labyrinthine symptoms of dizziness, nausea, and vertigo usually subside in 6 to 12 weeks (Table 3–2).4
 Injuries to the Glossopharyngeal, Vagus, Spinal Accessory, and Hypoglossal Nerves
Injuries to the Glossopharyngeal, Vagus, Spinal Accessory, and Hypoglossal Nerves
Cranial nerves IX, X, and XI, together with the internal jugular vein, pass through the jugular foramen at the base of the skull. The hypoglossal foramen, through which cranial nerve XII passes, lies just medial to the jugular foramen. Unilateral paralysis of the last four cranial nerves, which may be traumatic, inflammatory, or secondary to neoplastic compression, is referred to as the Collet-Sicard syndrome.12
Site of Trauma
Gunshot or stab wounds occasionally cause injury. A fracture of the occipital condyle, or Collet-Sicard syndrome, can injure all four nerves. The peripheral portion of CN XI can be injured in surgical procedures such as posterior cervical lymph node biopsies. The hypoglossal and recurrent laryngeal nerves can be traumatized in anterior neck operations such as carotid endarterectomy.1
Clinical Features
The symptomatology produced by an injury in this region can be inferred from the anatomy and function of the lower four cranial nerves. It consists of cardiac irregularities, excessive salivation, loss of sensation and gag reflex of the ipsilateral palate, loss of taste sensation of the posterior one third of the tongue, a hoarse voice with paralysis of the ipsilateral vocal cord, some dysphagia, and hemiatrophy of the tongue with deviation to the side of the injury. Additional symptoms and signs may be present if neighboring structures (e.g., the carotid artery, internal jugular vein, styloid process, and facial nerve) are also involved in the traumatic process.4
Lower cranial nerve findings associated with signs of brainstem compression are consistent with an intracranial lesion, whereas the presence of Horner’s syndrome is consistent with an extracranial lesion.1
Evaluation
A careful clinical examination is mandatory. CT and MRI scans are both useful, depending on the case.
Management
Treatment of Collet-Sicard syndrome is supportive with elevation of the head for drainage of excess saliva and intravenous (IV) or nasogastric nutrition until normal swallowing returns. Accessory nerve injuries in the neck may require exploration with neurolysis or resection and repair or grafting, depending on the degree of injury.1
Prognosis
Collet-Sicard syndrome may show slow partial recovery. Patients with vagal, spinal accessory, and hypoglossal nerve injuries associated with carotid endarterectomy often recover.4
< div class='tao-gold-member'>