INTRODUCTION
This chapter reviews congenital and acquired heart disease in children. The section on congenital heart defects begins with a review of fetal and neonatal cardiac physiology, followed by a discussion of specific lesions and their diagnosis and management organized by clinical presentation. A brief discussion of pediatric murmurs follows, and the section concludes with a discussion of common surgical procedures for repairing congenital heart defects and associated complications.
The section on acquired heart disease in children reviews inflammatory and infectious disorders and cardiomyopathies, of which the most important is hypertrophic cardiomyopathy.
PEDIATRIC CARDIAC PHYSIOLOGY
Fetal circulation involves a number of shunts to bypass the liquid-filled lungs, which are incapable of providing oxygen to circulating blood. Blood oxygenated by the maternal lungs passes through the placenta via the umbilical vein to the fetus. Roughly half of the blood flow passes through the liver and half through the ductus venosus to the inferior vena cava, where it mixes with deoxygenated fetal blood returning from the lower body. The inferior vena cava enters the right atrium, where deoxygenated blood returning from the upper body and head via the superior vena cava mixes with that from the inferior vena cava. Blood from the right atrium travels in one of three routes: A portion passes through the foramen ovale into the left atrium, where it subsequently travels to the left ventricle, through the aorta, and into the vessels supplying the fetal head and upper extremities; most of the remainder enters the right ventricle and passes into the pulmonary artery, where the majority is shunted away from the lungs through the ductus arteriosus connecting the pulmonary artery with the aorta; and a small amount of blood travels to the lungs to provide oxygen and nutrients to support fetal lung growth.1
The newborn’s lungs expand and become air filled with gradual reabsorption of fetal lung fluid. This increases the partial pressure of arterial oxygen (PaO2) of blood flowing through the newborn lung, which in turn mediates a cascade of events that completes the transition to adult circulatory patterns. Flow through the umbilical arteries ceases, and the venous flow through the cord slows and then stops. Pulmonary vascular resistance falls, and pulmonary blood flow increases (pulmonary vascular resistance continues to fall with increases in blood flow over the first 30 to 45 days of extrauterine life). The ductus venosus and ductus arteriosus close, and decreased pulmonary arterial resistance coupled with increased systemic resistance create increased blood flow through the atria. Left atrial pressure exceeds right atrial pressure, which leads to closure of the foramen ovale.
Because neonates and small children have relatively noncompliant ventricular walls, they cannot increase stroke volume but rely on changes in heart rate to adjust cardiac output. Thus, sinus tachycardia is usually the first response to stress in infants and young children. The neonatal myocardium requires more oxygen than the infant’s or child’s heart and has a lower systolic reserve, which predisposes to congestive heart failure. Although the ductus arteriosus and foramen ovale are usually functionally closed by 15 hours of life and 3 months of age, respectively, shunting may still occur through these pathways during times of stress.2 Finally, the neonatal right ventricle is still predominant, whereas the left ventricle is predominant in older infants and children, and pulmonary vascular resistance is relatively high and oxygen responsive.
Preload is the amount of blood that the heart receives to distribute to the body. Decreasing the amount of blood flowing into the heart lowers cardiac output. Similarly, increasing the amount of blood into the heart increases cardiac output in accordance with Starling forces, to the point of maximum compliance of the ventricular wall. When the ventricular wall compliance is exceeded, cardiac output decreases dramatically and congestive heart failure occurs.
Afterload is the resistance to blood flow out of the heart and is determined in neonates by the size and compliance of the ventricles, peripheral vascular resistance (which is largely mediated by catecholamines), and, when present, anatomic obstructions such as aortic stenosis or critical coarctation of the aorta.
Contractility or inotropy, which is the ability of the cardiac muscle to pump blood out of the heart, refers to the force or power of cardiac contraction and determines the amount of work that the heart can perform. Increasing the cardiac contractility increases the stroke volume and hence the cardiac output. Cardiac contractility is normally regulated by neural or humoral mechanisms. The ability of the neonatal heart to increase contractility is limited, as previously mentioned, and stroke volume is primarily increased through increases in rate.
Cardiac rate or chronotropy is the ability of the heart muscle to pump blood out of the heart per fixed unit of contraction. In the typical circumstance, chronotropy and inotropy cannot be differentiated with regard to therapeutic maneuvers. Typically, both the cardiac rate and the relatively fixed contractility of the neonatal heart contribute to the overall cardiac output, with the former contributing more of the output. In the hearts of children older than 4 or 5 years, there is a more balanced contribution to cardiac output, with the contractility playing a much more prominent role.
CONGENITAL HEART DISEASE
Congenital heart defects can present at different ages with clinical signs and symptoms ranging from cyanosis to cardiovascular collapse or congestive heart failure depending on the anatomy and physiology of the lesion. Long-term survivors are at risk for a number of postoperative complications.
Congenital heart defects occur in approximately eight in 1000 births and range from benign to life threatening. About 10% of congenital heart defects are associated with genetic syndromes such as trisomy 21, Turner’s syndrome, and Noonan’s syndrome, and heart defects may accompany other organ malformations in conditions such as VACTERL association (vertebral anomalies, anal atresia, cardiac anomalies, tracheoesophageal fistulas, esophageal atresia, renal and limb anomalies, and single umbilical artery). The remaining 90% of congenital heart defects result from isolated embryologic malformation or as yet undefined genetic lesions.
Congenital heart disease is usually classified based on physiology (presence or absence of cyanosis, with or without persistent fetal circulation) or on the nature of the anatomic defect (shunt, obstruction, transposition, or complex defect). Most textbooks separate cyanotic from acyanotic lesions.
Cyanotic lesions result in mixing of deoxygenated and oxygenated blood or right-to-left shunting; cyanotic lesions include the “five Ts“: tetralogy of Fallot, tricuspid anomalies including tricuspid atresia and Ebstein’s anomaly, truncus arteriosus, total anomalous pulmonary venous return, and transposition of the great arteries. Acyanotic lesions include those that result in pulmonary overcirculation such as ventricular septal defect, atrial septal defect, patent ductus arteriosus, and atrioventricular canal as well as those with restricted pulmonary or systemic blood flow such as pulmonary stenosis, aortic stenosis, and aortic coarctation.
It is often more useful to organize congenital heart disease by clinical presentation (Table 126-1). Distinct clinical presentations are discussed further in later sections, including the pathophysiology, clinical features and treatment, and individual defects within each group. Discussion of murmurs and arrhythmias included in this chapter is limited to those related to congenital heart disease. Rhythm disturbances are discussed in greater detail in the later section, “Acquired Heart Disease,” and syncope and sudden death are discussed in chapter 127, Syncope, Dysrhythmias, and ECG Interpretation in Children.
| Clinical Presentation | Causative Conditions in Neonates | Causative Conditions in Infants and Children |
|---|---|---|
| Cyanosis | Transposition of the great arteries, TOF, tricuspid atresia, truncus arteriosus, total anomalous pulmonary venous return | TOF, Eisenmenger’s complex |
| Cardiovascular shock | Critical AS, coarctation of the aorta, HLHS | Coarctation of the aorta (infants) |
| Congestive heart failure | Rare: PDA, HLHS | PDA, VSD, ASD, atrioventricular canal |
| Murmur | PDA, valvular defects (AS, PS) | VSD, ASD, PDA, outflow obstructions, valvular defects (AS, PS) |
| Syncope | — | AS, PS, Eisenmenger’s complex |
| Hypertension | — | Coarctation of the aorta |
| Arrhythmias | — | ASD, Ebstein’s anomaly, postsurgical complication after repair of congenital heart defect |
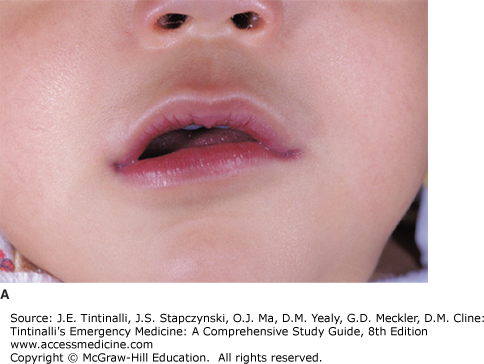
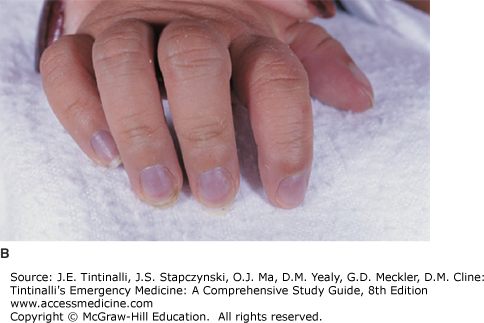
Cyanosis is the bluish discoloration of the skin that occurs from the presence of deoxygenated hemoglobin (which is blue) in capillary beds. For cyanosis to be clinically apparent, 3 to 5 milligrams/dL of deoxyhemoglobin must be present, corresponding to an oxygen saturation of 70% to 80% on room air.3,4,5 Compression of the placenta during birth typically leads to polycythemia in term newborns, and, as a result, clinical cyanosis develops more readily in newborns because a smaller percentage of circulating hemoglobin must be desaturated to manifest this sign.
Congenital heart defects that present with cyanosis include transposition of the great arteries, tetralogy of Fallot, tricuspid atresia, truncus arteriosus, and total anomalous pulmonary venous return. These lesions have in common the mixing of oxygenated and deoxygenated blood, circulation of desaturated hemoglobin, and a cardinal manifestation as cyanotic heart disease. Another condition resulting in cyanosis is persistent fetal circulation, which can be caused by structural heart disease or noncardiac disease, including meconium aspiration, pneumonia, sepsis, and pulmonary hypertension. Lesions that restrict pulmonary blood flow, such as critical pulmonary stenosis, do not typically cause cyanosis without the presence of associated defects (atrial septal defect, ventricular septal defect) that allow for right-to-left shunting.
Congenital heart defects can lead to cyanosis in the first weeks of life or, for some lesions, episodically throughout childhood if uncorrected. Lesions such as transposition of the great arteries are associated with mixing of oxygenated and deoxygenated blood, usually through an associated ventricular septal defect or atrial septal defect, and produce cyanosis in the period immediately after birth. Conditions associated with persistent pulmonary hypertension allow blood to shunt right to left through a patent foramen ovale or through a septal defect. Tetralogy of Fallot (see the next section, “Tetralogy of Fallot“) can produce cyanosis at birth through mixing, but is also associated with episodic cyanosis (“tet spells“; see the later section, “Treatment of Tet Spells“) throughout infancy and childhood if uncorrected. Large uncorrected septal defects (e.g., ventricular septal defect) can cause cyanosis in adolescents and young adults in a condition termed Eisenmenger’s complex. Chronic left-to-right shunting across a nonrestrictive defect leads to hypertrophy of pulmonary arteriolar musculature that causes a gradual and irreversible rise in pulmonary vascular resistance and right-sided heart pressures until supersystemic pressures develop and shunting switches to right to left, which produces cyanosis.
Noncardiac causes of cyanosis range from benign peripheral vasoconstriction in response to cold or crying, causing peripheral cyanosis, to sepsis with poor perfusion or even effects of toxins such as methemoglobin.6 The most common congenital heart defects that must be considered in the cyanotic neonate are briefly reviewed in the sections below before returning to a general approach to the evaluation and management of cyanotic congenital heart disease.
Tetralogy of Fallot is the most common cyanotic congenital heart disease manifesting in the postinfancy period and comprises as much as 10% of all congenital heart disease.7,8,9,10 There are four primary components of tetralogy of Fallot: a large ventricular septal defect, right ventricular outflow obstruction (created by valvular or supravalvular pulmonic stenosis), an overriding aorta, and right ventricular hypertrophy.
The intensity of cyanosis depends on the amount of obstruction of the right ventricular outflow tract. A nonrestrictive ventricular septal defect balances the systolic pressures in the right and left ventricles. The amount of right ventricular outflow obstruction determines whether shunting is left to right, bidirectional, or right to left. Severe pulmonic stenosis creates a right-to-left shunt, resulting in cyanosis and decreased pulmonary blood flow. The acyanotic form of tetralogy of Fallot is characterized by mild pulmonic stenosis with a left-to-right shunt. In addition to cyanosis, examination findings can include a systolic thrill at the lower and middle left sternal border. A loud single S2, an aortic ejection click, a loud systolic ejection murmur (heard best at the middle to lower left sternal border), and a continuous patent ductus arteriosus murmur may also be apparent on examination.
Comprising about 5% to 8% of all congenital heart disease, transposition of the great arteries is the most common cyanotic heart lesion manifesting in the newborn period. Compared to other congenital heart defects, extracardiac anomalies occur less often in babies with transposition of the great arteries (<10%).11 There are many variations in transposition of the great arteries, but the underlying elements are that the aorta arises from the right ventricle and the main pulmonary artery originates from the left ventricle. This arrangement gives rise to two distinct circulatory systems. Because the main pulmonary artery has higher oxygen saturation than the aorta, hyperoxic blood goes through the pulmonary system and hypoxic blood flows through the systemic system. Mixing of the two circulatory systems is the only manner in which oxygenated blood enters the systemic blood flow. A ventricular septal defect, atrial septal defect, or patent ductus arteriosus must exist in order for the infant to survive. In 20% to 40% of patients, a ventricular septal defect is present. The physical examination is noTable for a loud and single S2, and if a ventricular septal defect exists, a systolic murmur may be heard.
Total anomalous pulmonary venous return represents 1% of congenital heart disease.12 The pulmonary veins empty into the right atrium instead of returning blood from the lungs into the left atrium. Total anomalous pulmonary venous return is usually separated into four groups depending on where the pulmonary veins empty. In the supracardiac type (50% of all total anomalous pulmonary venous return cases), the common pulmonary vein is attached to the superior vena cava. In the cardiac type (20%), the common pulmonary vein drains into the coronary sinus. In the infracardiac/subdiaphragmatic type (20%), the common pulmonary vein empties into the portal vein, ductus venosus, hepatic vein, or inferior vena cava. Mixed lesions comprise the remaining 10%. Survival depends on the mixing of blood, so an atrial septal defect or a patent foramen ovale must be present.
When pulmonary venous return arrives in the right atrium, there is mixing of the pulmonary and systemic circulations. In the right atrium, blood crosses the atrial septal defect to the left atrium or crosses the tricuspid valve to the right ventricle. Systemic arterial blood becomes desaturated because of the mixing of pulmonary and systemic arterial flow. Pulmonary blood flow determines the degree of desaturation of systemic arterial blood. If there is no obstruction to pulmonary venous return, systemic blood is minimally desaturated. Obstruction to pulmonary venous return results in severe cyanosis. Because of the extra volume returning to the right side of the heart, right ventricular and atrial enlargement can develop.
Although total anomalous pulmonary venous return more commonly presents with signs and symptoms of congestive heart failure (see later section, “Congestive Heart Failure in Congenital Heart Disease“), tachypnea, tachycardia, hepatomegaly, and cyanosis are commonly seen. Children with pulmonary venous obstruction often have a history of frequent pneumonias and growth retardation. The physical examination reveals a right ventricular heave and fixed split S2. A grade 2/6 to 3/6 systolic ejection murmur heard at the left upper sternal border and a mid-diastolic rumble at the left lower sternal border are also heard. Total anomalous pulmonary venous return with pulmonary venous obstruction leads to respiratory distress and cyanosis with a loud and single S2 and a gallop but, on most occasions, no murmur.
Tricuspid atresia represents 1% to 2% of congenital heart disease.13 There is no tricuspid valve, and the development of the right ventricle and pulmonary artery is interrupted. Pulmonary blood flow is decreased. With no flow existing between the right atrium and right ventricle, an atrial septal defect, ventricular septal defect, or patent ductus arteriosus is necessary for survival because the right atrium requires a right-to-left shunt in order to empty. The great arteries are transposed, with a ventricular septal defect and pulmonic stenosis in 30% of cases. Artery anatomy is normal, with a small ventricular septal defect and pulmonic stenosis in half of cases.
With all of the systemic venous return shunted from the right atrium to the left atrium, right atrial dilatation and hypertrophy occur. Increased volume from the systemic and pulmonary circulations causes enlargement of the left atrium and left ventricle. The extent of cyanosis and the amount of pulmonary blood flow are inversely related.
Usually patients have marked cyanosis, tachypnea, and poor feeding. A single S2 is evident, as well as a grade 2/6 or 3/6 regurgitant systolic murmur heard best at the left lower sternal border. The continuous murmur of a patent ductus arteriosus may also exist. Hepatomegaly is present if there is congestive heart failure.
In truncus arteriosus, all of the pulmonary, systemic, and coronary circulations originate from a single arterial trunk. The defect comprises <1% of all congenital heart disease.14 Associated with truncus arteriosus are a large ventricular septal defect, coronary artery irregularities, and DiGeorge’s syndrome (hypocalcemia, hypoparathyroidism, absent or hypoplastic thymus, and chromosomal abnormalities). Pulmonary blood flow is determined by the type of truncus, and flow can be normal, increased, or decreased. There is a direct relationship between the amount of pulmonary blood flow and systemic arterial oxygen saturation. Decreased pulmonary blood flow creates marked cyanosis, whereas increased pul-mo-nary blood flow produces minimal cyanosis but is associated with congestive heart failure from left ventricular volume overload.
Congestive heart failure and cyanosis typically develop within the first few weeks of life. A loud regurgitant 2/6 to 4/6 systolic murmur at the left sternal border may be accompanied by a high-pitched diastolic decrescendo murmur or diastolic rumble. A single S2 is prominent.
The cardinal clinical presentation of cyanotic congenital heart defects is cyanosis. The history taking should elicit details of the pregnancy, gestational age, fetal US results if applicable, and complications of labor and delivery, including cyanosis in the period immediately after birth. For older infants and children with known congenital heart defects, details of the anatomy and surgical procedures and current medications should be obtained. Baseline oxygen saturations may be known by caretakers and are helpful when intercurrent illness leads to an ED visit. A careful feeding history should be obtained, focusing on changes in oral intake, slow or difficult feeding, sweating with feeds, and growth, and a complete review of systems should be performed.
Measure all vital signs, including blood pressure in the upper and lower extremities. A difference in upper and lower extremity blood pressures may signal an obstructive lesion such as coarctation of the aorta (see later section, “Coarctation of the Aorta“). Document weight and growth parameters. Note if cyanosis is central (mucosal) or peripheral (acral, involving digits) (Figure 126-1). Listen for cardiac murmurs, noting location, timing, and loudness (see later section, Pediatric Murmurs), and a gallop or fixed splitting of S2 (characteristic of atrial septal defect). Palpate the chest for heaves, lifts, and thrills, and note surgical scars. Observations of the strength, quality, and symmetry of pulses help in the assessment of cardiac output. Hepatomegaly and splenomegaly suggest right-sided heart failure. Observe for signs of increased work of breathing, and auscultate for rales, which suggest congestive heart failure. Neonates with cyanosis secondary to congenital heart disease rarely have respiratory symptoms other than tachypnea. Neonates with lung disease producing cyanosis show respiratory distress, grunting, tachypnea, and retractions. Cyanotic infants with CNS disturbances or sepsis have apnea, bradycardia, lethargy, and seizures. Neonates with methemoglobinemia show minimal distress despite their cyanotic appearance. The neurologic examination includes observation and examination of muscle tone and mental status—irritability may be a symptom of hypoxemia. Performing a complete head-to-toe examination is important in the cyanotic patient without a known history of congenital heart defects to exclude noncardiac causes.
Laboratory tests are not typically helpful in the evaluation of cyanotic congenital heart defects, although they help exclude other causes of cyanosis. Results of the “hyperoxia test” (PaO2 in response to breathing 100% oxygen) may help distinguish heart disease from other causes of cyanosis. Neonates with cyanotic heart disease do not demonstrate an increase in PaO2 >20 mm Hg, because of the right-to-left shunting of the circulation. Most neonates with lung disease or sepsis, however, demonstrate an increase in PaO2 after breathing 100% oxygen for 20 minutes. Infants with persistent pulmonary hypertension may or may not demonstrate a significant rise in PaO2. There is no response to oxygen in the neonate with methemoglobinemia. When a blood specimen is exposed to air, it turns pink in all the conditions described above except in methemoglobinemia, in which the blood remains chocolate colored. In an infant without known congenital heart defects, results of arterial blood gas analysis with the infant breathing room air and 100% oxygen can be compared: Failure of the PaO2 to rise significantly with 100% oxygen suggests cardiac mixing or right-to-left shunting, whereas improvement in the PaO2 in response to oxygen suggests a pulmonary cause.
The primary diagnostic tests for the patient with suspected cyanotic congenital heart defects are chest radiography and electrocardiogram (Table 126-2). Chest radiographic studies are essential in assessing the size and shape of the heart and in evaluating pulmonary blood flow. The chest radiograph also provides some information about the position of the aortic arch, which should be normally left sided. In the normal left-sided aortic arch, there is rightward displacement of the esophagus and trachea. An abnormal position of the aortic arch may be a clue to the diagnosis of the congenital cardiac lesion. Right-sided aortic arches are seen in truncus arteriosus, transposition of the great arteries, tetralogy of Fallot, tricuspid atresia, and total anomalous pulmonary venous return. The chest radiograph is critical to the assessment of pulmonary vascularity. With small left-to-right shunts, the pulmonary vascularity is normal. Pulmonary vascularity can also be normal in conditions that cause pulmonary stenosis, such as valvular pulmonic stenosis or functional pulmonic stenosis associated with tetralogy of Fallot. Increased pulmonary vascularity may be seen with any cause of left-to-right shunting or in any cause of left-sided failure, such as outflow obstruction.
| Cardiac Lesion | Chest Radiograph | Electrocardiogram |
|---|---|---|
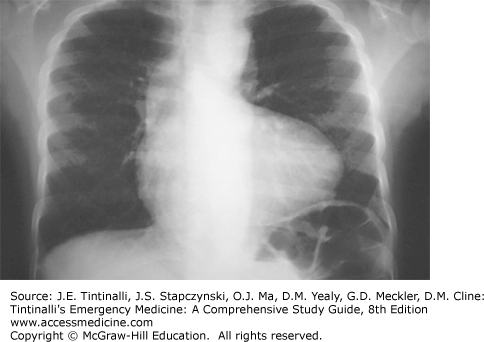
| Tetralogy of Fallot | Boot-shaped heart, normal-sized heart, decreased pulmonary vascular markings | Right axis deviation, right ventricular hypertrophy |
| Transposition of the great arteries | Egg-shaped heart, narrow mediastinum, increased pulmonary vascular marking | Right axis deviation, right ventricular hypertrophy |
| Total anomalous pulmonary venous return | Snowman sign, significant cardiomegaly, increased pulmonary vascular markings | Right axis deviation, right ventricular hypertrophy, right atrial enlargement |
| Tricuspid atresia | Heart of normal to slightly increased size, decreased pulmonary vascular markings | Superior QRS axis with right atrial hypertrophy, left atrial hypertrophy, left ventricular hypertrophy |
| Truncus arteriosus | Cardiomegaly, increased pulmonary vascular markings | Biventricular hypertrophy |
The electrocardiogram is useful to evaluate chamber size, electrical axis, and cardiac conduction. Age-related normal values should be used as a reference to determine axis deviation, atrial enlargement, or ventricular hypertrophy.15,16 The electrical axis most often defines abnormal chamber diameters and usually does not suggest cardiac ischemia as in the adult population. Table 126-2 lists characteristic chest radiograph and electrocardiogram findings of cyanotic congenital heart defects. Figure 126-2 depicts the typical “boot-shaped heart” of tetralogy of Fallot.
When available, bedside echocardiography may delineate structural heart disease, although adequate imaging depends on the availability of an ultrasonographer with pediatric cardiac experience.
The management of cyanotic congenital heart defects depends on the age of the patient, hemodynamic stability, and prior diagnosis and medical management. Most cyanotic congenital heart defects are hemodynamically stable. With obstructive lesions, in contrast, adequate circulation often depends on systemic or pulmonary blood flow through a patent ductus arteriosus, and such lesions can be fatal if patency is not maintained with prostaglandins (see later section, “Shock in Congenital Heart Disease“). Although central cyanosis and low oxygen saturation are alarming and may tempt one to administer oxygen immediately, neonates have significant amounts of oxygen-avid fetal hemoglobin and tolerate oxygen saturation percentages in the 70s (characteristically seen in most mixing lesions) without tissue or brain hypoxemia. Moreover, oxygen is a potent pulmonary vasodilator. Oxygen administration and pulmonary vasodilation are helpful in treating cyanotic congenital heart defects associated with pulmonary hypertension or vasoconstriction, but may actually lead to pulmonary vascular overcirculation or even “steal” of systemic blood flow in patients with a patent ductus arteriosus and ductal-dependent systemic blood flow. Oxygen administration should be reserved for patients with signs and symptoms of inadequate tissue perfusion, those without known heart disease in whom it may be diagnostic as well as therapeutic, and patients with known congenital heart defects with oxygen saturation significantly below known baseline values.
The primary management objective in the cyanotic neonate or infant is the treatment of intercurrent illness, exclusion of noncardiac causes of cyanosis, and diagnosis of cyanotic congenital heart defects in those who do not have a previous diagnosis. Treatment thereafter involves consultation with a pediatric cardiologist and transfer to a tertiary pediatric hospital or clinic.
A tet spell is caused by right-sided outflow tract obstruction leading to right-to-left shunting through a ventricular septal defect. Hypoxia and acidosis cause pulmonary arterial vasoconstriction, thus increasing pulmonary resistance and exacerbating shunting. The management goals for tet spells are to increase pulmonary blood flow by increasing preload, provide pulmonary vasodilation, and increase afterload in order to reverse right-to-left shunting and promote pulmonary blood flow. This is often achieved through simple maneuvers such as administering 100% oxygen via a non-rebreathing face mask, calming the child by minimizing stimulation and placing the child in a parent’s arms, and flexing the child’s knees to the chest in order to increase venous return to the heart and increase systemic vascular resistance to mitigate right-to-left shunting. Second-line intervention includes administration of morphine, 0.1 to 0.2 milligram/kg IM, SC, or IV, and isotonic fluid (normal saline 20 mL/kg bolus) to increase preload. If these measures are unsuccessful, next options include administration of sodium bicarbonate 2 mEq/kg as an IV bolus to treat acidosis and promote pulmonary vasodilation; propranolol 0.2 milligram/kg IV to relieve infundibular spasm; or phenylephrine 2 to 10 micrograms/kg/min to increase systemic vascular resistance. Refractory spells may require neuromuscular blockade and rapid-sequence intubation.
The presentation of congenital heart defects as shock or cardiovascular collapse is dramatic. Cardiogenic shock can be the final common pathway for a wide variety of disease processes, both noncardiac and cardiac. Sepsis, hypovolemic shock, metabolic disease, adrenal insufficiency, respiratory failure, trauma, and poisonings can all lead to cardiogenic shock and are discussed in other chapters. Consider noncardiac causes of shock and low cardiac output states, and treat the patient accordingly while contemplating the possibility of congenital heart disease.
Congenital heart defects that present as shock or cardiovascular collapse include lesions that depend on flow through the ductus arteriosus to provide systemic or pulmonary perfusion. The classic examples are severe coarctation of the aorta and hypoplastic left heart syndrome. In both of these conditions, systemic blood flow is restricted by the underlying defect but maintained by flow through a patent ductus arteriosus that bypasses the coarctation in the former and allows blood pumped by the functional right ventricle to perfuse the aorta in the latter. The ductus typically closes and becomes the ligamentum arteriosum by the second or third week of life, and as it constricts, patients with duct-dependent flow manifest poor peripheral perfusion with increasing acidosis and eventual cardiovascular collapse.
Once the ductus arteriosus begins to close, some cardiac lesions become incompatible with life, because blood can no longer reach the lungs or distal circulation. Both cyanotic and acyanotic lesions may present in this fashion.
Acyanotic lesions include severe coarctation of the aorta, critical aortic stenosis, and a hypoplastic left ventricle. Transposition of the great arteries, pulmonary atresia, and hypoplastic right heart syndrome are examples of the cyanotic lesions that may present with shock.
Nonstructural cardiac causes of shock include dysfunctional myocardium, which may mimic the signs and symptoms seen with shunt-dependent anatomic lesions. Such cardiomyopathies are uncommon in pediatric patients but can easily be confused with anatomic lesions. Cardiomyopathies in children are discussed later in the “Acquired Heart Disease” section.
Patent duct arteriosis–dependent acyanotic congenital lesions are briefly reviewed in the following sections.
Coarctation of the aorta represents 8% to 10% of congenital heart disease and has a 2:1 male predominance. Congenital narrowing of the aorta takes place around the ductus arteriosus in the upper thoracic aorta. Factors determining the severity and clinical manifestations of disease include the degree of narrowing, the length of narrowing, and the presence of associated defects. Infants who present early have a right ventricle that supplies the descending aorta through a patent ductus arteriosus in fetal life. A ventricular septal defect, patent ductus arteriosus, aortic hypoplasia, and underdeveloped collateral circulation can also be seen.
A patent ductus arteriosus delays the obstructive effects of coarctation by allowing blood to flow distal to the obstruction. With closure of the patent ductus arteriosus, pulmonary hypertension occurs, leading to pulmonary venous congestion and congestive heart failure. Blood flow distal to the aortic obstruction is compromised. Shock, metabolic acidosis, tachypnea, and feeding difficulty are common; when congestive heart failure occurs, a loud gallop and weak pulses with or without a murmur can usually be appreciated.
Finding decreased pulses in the lower extremities is essential to diagnosing a coarctation. Comparing right upper extremity blood pressures and pulse oximeter readings with those of the lower extremities aids in diagnosis unless the patient is in shock, in which case pulses may be decreased all over.
Hypoplastic left heart syndrome consists of hypoplasia of the left ventricle and ascending aorta and aortic arch. Atresia or marked stenosis of the mitral and aortic valves and regressed development of the left atrium are also common. These combined lesions lead to minimal left ventricular outflow. In utero pulmonary vascular resistance remains higher than systemic vascular resistance. The right ventricle is able to maintain normal perfusion of the body through right-to-left shunting through the patent ductus arteriosus as a result of elevated pulmonary resistance. Systemic blood flow is based entirely on the ductus arteriosus. After birth, major problems occur as reversal of the fetal pulmonary-systemic pressure gradient takes place and the ductus arteriosus closes. Cardiac output collapses and aortic pressure falls, which results in circulatory shock and metabolic acidosis. Pulmonary edema also develops because of increased pulmonary blood flow and increased left atrial pressure. Signs at presentation include an ashen gray color, tachypnea, and listlessness, and a single heart sound, systolic ejection murmur, and decreased pulses are noted.17,18
Aortic stenosis comprises 6% of congenital heart disease and has a 4:1 male predominance. Stenosis can occur at the valvular, supravalvular, or subvalvular levels. Infants with severe obstruction (10% to 15%) present with congestive heart failure and poor distal perfusion or shock. Left ventricular hypertrophy typically develops in severe stenosis. Patients with aortic stenosis who are asymptomatic in infancy can present in childhood with syncope and hypertension.19,20,21
A bicuspid aortic valve is the most common form of aortic stenosis. Supravalvular aortic stenosis, elfin facies, mental retardation, and pulmonary artery stenosis comprise Williams’ syndrome. A systolic thrill may be noticed at the right upper sternal border, suprasternal notch, or carotid arteries along with an ejection click. There can also be a rough or harsh grade 2/6 to 4/6 systolic murmur at the right or left sternal border with transmission to the neck.
The typical history of the neonate with duct-dependent congenital heart defects is one of a day or two of poor feeding, irritability, or lethargy followed by decreasing responsiveness, typically in the second or third week of life. By the time the neonate arrives in the ED, he or she is often in severe shock. A complete history of the pregnancy, labor and delivery, and immediate perinatal period should be obtained and symptoms should be reviewed to rule out noncardiac causes of shock such as vomiting, diarrhea, fever, and respiratory distress.
Assessment of vital signs should include four-extremity blood pressure measurement to identify a gradient between upper and lower extremities characteristic of coarctation of the aorta. Pulse oximetry measurements in the right (preductal) and left (postductal) upper extremities may also reveal a difference suggesting duct-dependent flow. Tachycardia is usually severe, and tachypnea may reflect profound metabolic acidosis or may be a manifestation of heart failure. Hypoxemia and cyanosis may accompany cyanotic lesions with duct-dependent flow. An ashen or gray color is characteristic of the infant with left-sided outflow obstruction in systemic shock, and extremities may be cold and mottled with severely delayed capillary refill. A single heart sound is characteristic of hypoplastic left heart syndrome, and a harsh systolic murmur transmitted to the neck may be heard in patients with aortic stenosis. A gallop rhythm may be appreciated when congestive heart failure accompanies shock. Pulses are typically thready and may be absent in the lower extremities with significant delay between right brachial and femoral pulses. The lung examination may reveal rales, tachypnea, and retractions or grunting in neonates with both shock and congestive heart failure. The infant may be limp and lethargic.
Laboratory studies that may aid in the diagnosis and management of duct-dependent congenital heart defects include arterial blood gas analysis, which often demonstrates profound metabolic acidosis. Other electrolyte abnormalities are rare, although renal insufficiency from hypoperfusion may accompany severe shock. A CBC is not routinely helpful but may be obtained to rule out noncardiac causes of shock, such as sepsis.
Electrocardiogram and chest radiography are typically performed and may be useful in narrowing the differential diagnosis of suspected congenital heart defects. Table 126-3 lists the characteristic findings in duct-dependent acyanotic lesions.
| Cardiac Lesion | Chest Radiograph | Electrocardiogram |
|---|---|---|
| Coarctation of the aorta | Cardiomegaly with pulmonary edema (neonate) Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|