Figure 3.1. Lack of correlation between patient weight and peak plasma concentration after epidural administration of 400 mg of lignocaine. This same lack of a relationship between patient weight and peak plasma concentration has been demonstrated for multiple local anesthetics and different types of block. (Redrawn from Braid DP, Scott DB. Dosage of lignocaine in epidural block in relation to toxicity. Br J Anaesth 1966;38:596.)
7. Among esters, chloroprocaine and procaine are least likely to cause systemic toxic reactions because of their relatively rapid metabolism by plasma cholinesterase. Among amides, prilocaine is least likely to cause systemic toxicity because of its relatively extensive redistribution (large volume of distribution) and relatively rapid hepatic metabolism.
8. Because of their much slower absorption, hydrophobic drugs like bupivacaine and etidocaine are less likely to cause systemic toxicity than lidocaine if intravascular injection is avoided.
9. Toxicity is additive when multiple local anesthetics are used, that is, mixing two different local anesthetics does not reduce the risk of toxicity.
10. Often, one local anesthetic enantiomer is less toxic than the other. For example, the levorotary isomer of bupivacaine is less toxic than the dextrorotary isomer.
B. CNS toxicity
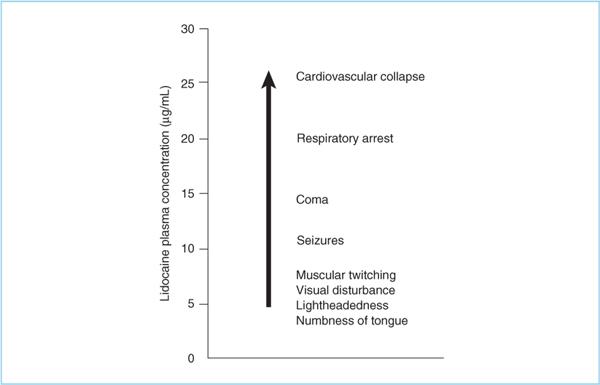
1. If local anesthetic plasma concentrations rise slowly enough (e.g., absorption from tissues), patients progress through a reproducible series of CNS signs and symptoms (23) (Figure 3.3). With a rapid increase in plasma concentrations (e.g., intravascular injection), seizures may be the first manifestation.
2. The therapeutic to CNS toxicity ratio is the same for all local anesthetics, that is, there is no difference in their propensity to cause seizures.
3. Sedative-hypnotic (e.g., benzodiazepine, propofol, and barbiturate) premedication raises seizure threshold (24) and probably prevents many seizures that would otherwise occur in patients not receiving sedative/hypnotic premedication.
4. Treatment. Treatment consists primarily of airway management to prevent hypoxia and cardiovascular support, if necessary, until plasma concentrations fall below the seizure threshold. Importantly, the hypercarbia and acidosis produced by seizures displaces local anesthetics from plasma protein binding sites, thereby increasing the free plasma concentration and potentially worsening toxicity (e.g., prolonged seizures, cardiovascular toxicity). Consequently, there is potential value in stopping the tonic/clonic convulsion. Seizures can be rapidly terminated with a sedative hypnotic (e.g., benzodiazepine, barbiturate) or the motor component (which is the source of the hypercarbia and metabolic acidosis) with a muscle relaxant (succinylcholine is most rapid).
C. Cardiovascular toxicity
1. The very high local anesthetic plasma concentrations necessary to cause significant cardiovascular toxicity can probably only be reached by intravascular injection.
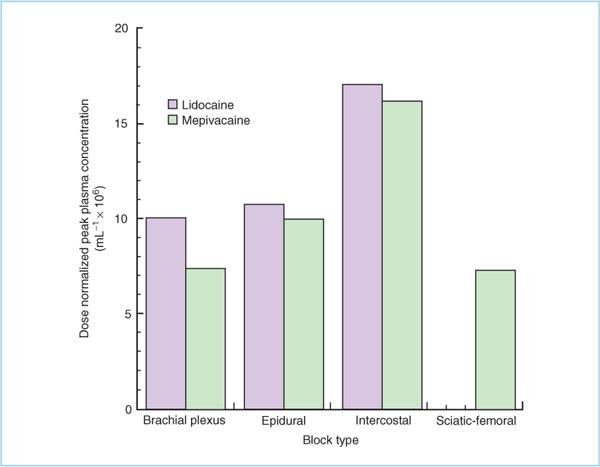
Figure 3.2. Dose-normalized peak plasma concentrations of lidocaine and mepivacaine following different types of nerve block. Highest concentrations occur following intercostal blocks.
2. The therapeutic/cardiotoxic ratio is lower for hydrophobic local anesthetics (e.g., etidocaine, bupivacaine) than for more hydrophilic drugs.
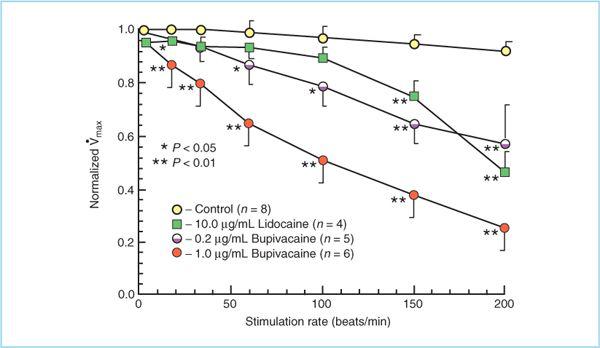
a. The difference in relative myocardial toxicity between hydrophilic and hydrophobic local anesthetics is, at least in part, the result of rate-dependent block. That is, between myocardial contractions local anesthetics can diffuse away from their binding sites in myocardial sodium channels so that when the next depolarization occurs the sodium channel can conduct Na+ normally. Because hydrophilic local anesthetics require less time to dissociate from the sodium channel binding site, it is more likely that myocardial sodium channels will function normally at physiological heart rates (HRs) when exposed to hydrophilic local anesthetic than to hydrophobic ones (Figure 3.4).
3. Cardiovascular toxicity is manifest as either malignant dysrhythmias, including ventricular fibrillation, and/or pulseless electrical activity (PEA) (24–26).
4. Treatment
a. Dysrhythmis should not be treated with lidocaine or any other local anesthetic. Amiodarone has not been extensively studied but is probably the best choice for treatment of serious local anesthetic–induced dysrhythmias.
Figure 3.3. The signs and symptoms of local anesthetic toxicity progress through a fairly stereotypical sequence regardless of local anesthetic used, as long as plasma concentrations rises relatively slowly. A very rapid increase in plasma concentration may result in “skipping” of some signs and symptoms. Premedication, especially with sedative hypnotics, may modify the sequence (e.g., deay seizures) or obscure the patient’s ability to report symptoms.
b. Animal studies demonstrate that standard advanced cardiac life support (ACLS) protocols are inadequate to treat local anesthetic–induced PEA. Much larger and more frequent dosing with epinephrine is required (24–27). Calcium should also be considered to help counteract the profound vasodilatation and impaired contractility produced by the very high local anesthetic plasma concentrations associated with cardiovascular toxicity. Bicarbonate may be useful in the setting of metabolic acidosis because it will help prevent displacement of local anesthetic from plasma protein binding sites.
c. Intralipid. Animal studies (28,29) and limited human experience (30) demonstrate that intralipid is an effective treatment for local anesthetic–induced cardiovascular toxicity. In vitro studies in isolated hearts suggest that intralipid changes the hydrophobic character of blood so that hydrophobic local anesthetics partition from the myocardium back into the plasma (31).
(1) Dose. 1 mL/kg bolus of 20% intralipid followed by 0.25 mL/kg/min infusion. Bolus may be repeated twice. Maximum total dose not to exceed 8 mL/kg.
(2) Just as dantrolene is kept on hand to treat rare cases of malignant hyperthermia, current evidence suggests that it is reasonable to keep a bottle of intralipid on hand to treat local anesthetic–mediated cardiovascular toxicity.
Figure 3.4. Heart rate–dependent effects of lidocaine and bupivacaine on velocity of the cardiac action potential (Vmax). Bupivacaine progressively decreases Vmax at heart rates above 10 beats/min due to accumulation of sodium channel block, whereas lidocaine does not decrease Vmax until heart rate exceeds 150 beats/min. (Adapted with permission from Clarkson CW, Hondegham LM. Mechanisms for bupivacaine depression of cardiac conduction: fast block of sodium channels during the action potential with slow recovery from block during diastole. Anesthesiology 1985:62:396.)
5. Preventing systemic toxicity
a. Use the smallest local anesthetic dose possible.
b. Inject local anesthetics slowly and incrementally so that intravascular injection can be recognized before a toxic or fatal dose is administered.
c. Test dose. Use of a test dose to identify intravascular injection is arguably the single most important step to prevent CNS and cardiovascular toxicity (32). Multiple types of test dose have been evaluated, including:
(1) Drugs intended to produce a subjective CNS effect when accidentally administered intravenously, for example, local anesthetics, opioids. This approach works well in nonsedated subjects trained in what to expect, but midazolam premedication makes local anesthetic symptoms unreliable (32).
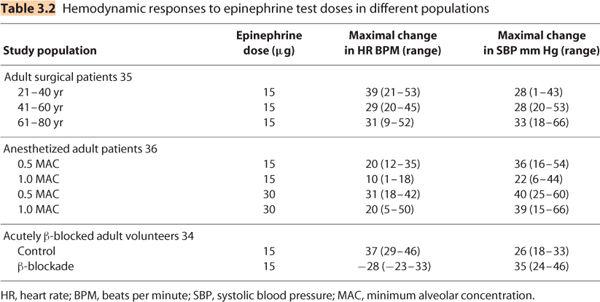
(2) Cardioactive drugs (e.g., epinephrine, norepinephrine) are added to local anesthetics because they produce objective cardiovascular effects (increased HR and/or blood pressure) when injected intravascularly (33). The recommended test dose is 3 mL of a local anesthetic solution containing 15 μg epinephrine (1:200,000 = 5 μg/mL). Guinard et al. have shown that a HR increase of 20 beats/min occurring within 2 minutes of administering this test dose is a 100% sensitive and 100% specific indicator of intravascular injection in young adults (34). Therefore, the 15-μg epinephrine test dose is an excellent indicator of intravascular injection if the following caveats are kept in mind (Table 3.2):
(a) The magnitude of the HR increase is reduced as patients age, particularly after the age of 40 (32). In fact, some elderly patients may not demonstrate an HR increase in response to 15-μg epinephrine. Blood pressure response is not significantly altered by age.
(b) The HR response is reduced, if not eliminated, in subjects who are acutely β-blocked (34). In this group, systolic blood pressure response (α1-adrenergic effect) is a better indicator of intravascular injection (Table 3.2).
(i) The effect of chronic β-adrenergic blockade on the hemodynamic response to an epinephrine-containing test dose is not known.
(ii) The effect of an epinephrine-containing test dose in patients taking β-blockers plus a vasodilating antihypertensive (e.g., angiotensin-converting enzyme [ACE inhibitor], angiotensin-II receptor blocker) is unknown.
(c) The HR and blood pressure response is reduced in anesthetized patients (32). As with acutely β-blocked subjects, systolic blood pressure is a better indicator of intravascular injection of an epinephrine-containing test dose than is HR increase in anesthetized patients (Table 3.2).
(3) T-wave changes. Reductions in T-wave amplitude (25% or 0.1 mV) have also been shown to be a reliable indicator of intravascular injection of an epinephrine-containing test dose (37). The shortcoming of this approach is that it is often difficult to adequately quantify T-wave changes “on-the-fly” using available electrocardiogram (ECG) monitors.
(4) Air. Air injected while listening for “mill-wheel” murmur over right atrium/ventricle has been shown to be an effective indicator of intravascular injection (32). This test is perhaps most useful in labor because contractions may produce hemodynamic changes that mimic an epinephrine-containing test dose.
The risk of allergic reaction to ester-type local anesthetics is low and to amide local anesthetics is extremely low (38). Many cases of local anesthetic “allergy” probably result from patients mistakenly attributing a side effect to an allergic reaction, for example, intravascular injection, epinephrine-induced tachyarrhythmias, vasovagal reactions, and so on.
A. Esters. Most allergic reactions to ester local anesthetics are probably reactions to their common metabolite, para-aminobenzoic acid (PABA). This explains why there is allergic cross-reactivity between different ester local anesthetics. Patients with a known allergy to PABA (common in cosmetics and sunscreens) should probably not receive ester local anesthetics.
B. Amides. Documented amide allergy is extremely rare. Allergic cross-reactivity between esters and amides probably does not occur.
C. Diagnosis. If an allergic reaction is suspected, blood can be drawn for measurement of plasma esterase, which is generally increased in the event of a “true” allergic reaction. Skin testing can be performed to prospectively identify patients with local anesthetic allergy (39).
V. Non–local anesthetic-mediated toxicity
Unintentional injection of toxic chemicals is an ever-present danger given the proximity of chemicals for skin prep (e.g., Betadine, chlorhexidine) and local anesthetics for nerve block. Similarly, other toxins in the operating room (OR) environment have accidentally been injected (e.g., formaldehyde for preserving biopsy specimens) causing injury and death. Another danger is the use of peripheral, epidural, or intrathecal catheters for continuous drug infusion. Everything you can imagine (propofol, thiopental, intralipid, antibiotics, muscle relaxants, etc.) has accidentally been injected through these catheters in the OR or on the surgical ward by persons mistaking the catheter for intravenous tubing; sometimes causing serious injury.
A. To reduce these risks:
1. Clearly label the catheter at the catheter’s connector.
2. Use tubing that does not resemble intravenous tubing and that lacks in-line access ports when connecting epidural or peripheral nerve catheters to infusion pumps.
3. Do not connect stopcocks to the catheter.
4. Continually educate nurses who are responsible for administering drugs in the hospital wards.
VI. Bleeding complications
At least some bleeding is probably a universal occurrence during peripheral and central neuraxial blocks. Bleeding that produces a hematoma during peripheral nerve blocks may make landmarks difficult to palpate but is generally not serious. However, epidural or intrathecal hematomas can cause devastating neurologic injury. The increased use of prophylaxis for thromboembolism in the perioperative period has increased this risk. The American Society of Regional Anesthesia and Pain Medicine has reviewed the risks attendant to performance of regional blocks in the anticoagulated patient and published guidelines (40) that are also posted on their website (www.asra.com), which should be considered the most current source of recommendations in this area.
A. Coagulopathy. Whether iatrogenic (e.g., heparin, Coumadin, platelet inhibitors, etc.), self-induced (e.g., ginkgo, garlic, and ginseng), or the result of a disease process, coagulopathy is probably the biggest risk factor for serious bleeding complications. Regional anesthesia, especially epidural and spinal anesthesia, should probably be avoided in fully anticoagulated patients unless there is a clear benefit that outweighs the added risk.
1. Nonsteroidal anti-inflammatory drugs (NSAIDS). In the absence of other coagulation defects, aspirin and other NSAIDS do not appear to significantly increase hematoma risk.
2. Low-dose unfractionated heparin. “Minidose” or thromboprophylactic subcutaneous heparin does not appear to increase the risk of epidural hematoma in twice-daily dosing.
3. Case reports suggest that epidural catheter removal may be as great a risk for epidural hematoma as catheter placement in the anticoagulated patient (41). Therefore, clinicians need to consider the possibility of intraoperative or postoperative anticoagulation before placing an epidural catheter.
a. It appears safe to place an epidural catheter in a patient who will subsequently be fully anticoagulated with heparin (e.g., cardiac or vascular surgery), under the following conditions (40):
(1) At least 1 hour elapses between catheter placement and anticoagulation.
(2) Care is taken to time catheter removal so that it is done when the patient’s coagulation status has normalized.
(3) Surgery is cancelled if a free aspiration of blood occurs. “Traumatic block” has been implicated as increasing the risk of hematoma, but it should be noted that the original recommendation for cancellation of surgery was applied to only 4 patients in the original series of 4,011 cases (42).
(4) Patients are not taking any other anticoagulant drugs, for example, NSAIDS.
B. Epidural/intrathecal hematoma. A rare but potentially devastating complication. Incidence estimated at less than 1:150,000 central neuraxial blocks.
1. Risk factors. In addition to anticoagulation, multiple attempts and/or traumatic (bloody) needle insertion is a feature of approximately 50% of reported cases.
2. Presentation. Most often presents as motor weakness and/or sensory loss, which can make it difficult to distinguish from block associated with continuous postoperative epidural analgesia. Back pain is not a universal presentation. Presentation is often more than 24 hours after block performed. Any onset of unexpected weakness is an indication for immediate neurologic evaluation and diagnostic imaging (magnetic resonance imaging [MRI] preferred, computed axial tomography [CT] scan acceptable), because urgent intervention is necessary.
3. Treatment. A few cases of successful conservative (nonoperative) treatment have been reported (43); however, expeditious (less than 8 hours from symptom onset) surgical evacuation of the hematoma is the treatment of choice. The rate and extent of recovery depends on how rapidly the hematoma is evacuated.
Infection is uncommon. Risk factors include immunocompromise, indwelling catheters, duration of indwelling catheter, and lack of perioperative antibiotics. Although not specifically studied, performing blocks through infected tissues probably increases the risk of further infectious complications and should be avoided.
A. Peripheral nerve blocks. The risk of infection caused by “single-shot” peripheral nerve blocks placed using appropriate aseptic technique is extremely low. The risk of infection or colonization increases when indwelling catheters are used. Still, although catheters are frequently colonized (approximately 70%; primarily Staphylococcus epidermidis), clinical evidence of infection is uncommon (less than 3%).
B. Central neuraxial blocks. The risk of infection from “single-shot” spinal and epidural anesthesia is low, although probably higher than for peripheral nerve blocks. Incidence of meningitis following spinal anesthesia is estimated at less than 1:40,000 and the risk of abscess following epidural anesthesia is estimated at less than 1:10,000 (2).
1. Risk factors
a. As with peripheral nerve catheters, epidural catheters increase the risk of epidural abscess.
b. Animal data suggest that untreated bacteremia increases the risk of meningitis following lumbar puncture. When appropriate antibiotic treatment was given, lumbar puncture did not increase the risk of meningitis in the setting of bacteremia (44). It is unknown if this is true in humans.
c. Chorioamnionitis. Studies to date suggest that chorioamnionitis in the peripartum period does not result in increased risk of infectious complications during regional anesthesia for labor analgesia.
C. Signs/symptoms of infection. Local tenderness, erythema, fever, and leukocytosis are to be expected with peripheral infections. Meningitis typically presents with fever, headache, photophobia, meningismus, and later altered mental status. Epidural abscess often presents with back and/or radicular pain, which may be indolent. Onset of sensory/motor changes may progress rapidly to paralysis.
D. Treatment. Peripheral catheter-related infections generally respond to catheter removal and appropriate antibiotic therapy as determined by culture. Epidural abscess and meningitis are medical emergencies with high morbidity/mortality and aggressive medical and/or surgical intervention is warranted.
REFERENCES
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree