CHAPTER 65
Commercially Available Reservoir Options for Intrathecal Drug Delivery
INTRODUCTION
The presence of abundant opioid mu receptors in the dorsal horns of the spinal cord was discovered in the 1970s. Subarachnoid injection of morphine was soon utilized to control cancer pain. Since intrathecal delivery of opioids provides direct access to the mu receptors, a much lower dose can achieve analgesia than by other routes of delivery. Thus, significant activation of systemic mu receptors, such as occur in the gut, is avoided thereby reducing unwanted side effects of opioid therapy such as constipation. The first clinical use of an implanted intrathecal drug delivery system (IDDS) was described in 1981. Introduction of effective bacteriostatic filters allowed safe long-term infusion. By 1991, commercially available fully programmable implanted IDDS became available (Medtronic, Inc, Minneapolis, MN).
Presently, commercially available pumps fall into two categories: continuous infusion and programmable rate devices. Several continuous infusion devices have been approved by the FDA (eg, Codman 3000, Codman and Shurtleff, Inc, a Johnson & Johnson Company, Raynham, MA, US). At present, the only programmable IDDS approved for pain indications in the US is the SynchroMed family of pumps (Medtronic, Inc, Minneapolis, MN).
CONTINUOUS INFUSION PUMPS
Fundamentals
• Continuous infusion pumps are typically designed as two compartment devices.
1. Inner compartment containing the drug to be administered
2. Outside chamber, which is filled with a 2-phase fluorocarbon propellant, which applies continuous pressure to the inner compartment.
• Filling the drug reservoir compresses the propellant and this pressure drives the drug from the collapsible chamber through a flow restrictor and then into the patient’s spinal catheter.
• Flow rates are factory preset.
• The advantage of a continuous flow rate device is that it does not require an energy source or complex programming circuitry and therefore does not need replacement because of battery life or utilize a specialized programmer.
• The main disadvantage is that any dosage adjustment requires emptying and refilling the pump with a newly formulated drug concentration. Additionally, potential clinical advantages such as timed bolus dosing, varying flow rates, and on-board storage of clinical data are not possible.
There are several mechanical, continuous infusion pumps that have been available in the United States. For example, Infusaid (Pfizer Hospital Products Group, Inc) and Isomed (Medtronic, Inc) are no longer available (Figure 65-1). At the present time, the Codman 3000 (Codman & Shurtleff, Inc, Raynham, MA) formerly known as Arrow 3000, and prior to that as Therex 3000, is the only approved mechanical pump in the United States (Figure 65-2).

Figure 65-1. Medtronic IsoMed pump.

Figure 65-2. Codman 3000—16 mL (left), 30 mL (center), and 50 mL (right).
CODMAN 3000 INFUSION PUMP
Indications
• Continuous regional delivery of intraspinal (intrathecal or epidural) preservative free morphine or baclofen
• Hepatic artery infusion for cancer treatment
Specifications
See Table 65-1.
TABLE 65-1. Specifics of Codman 3000
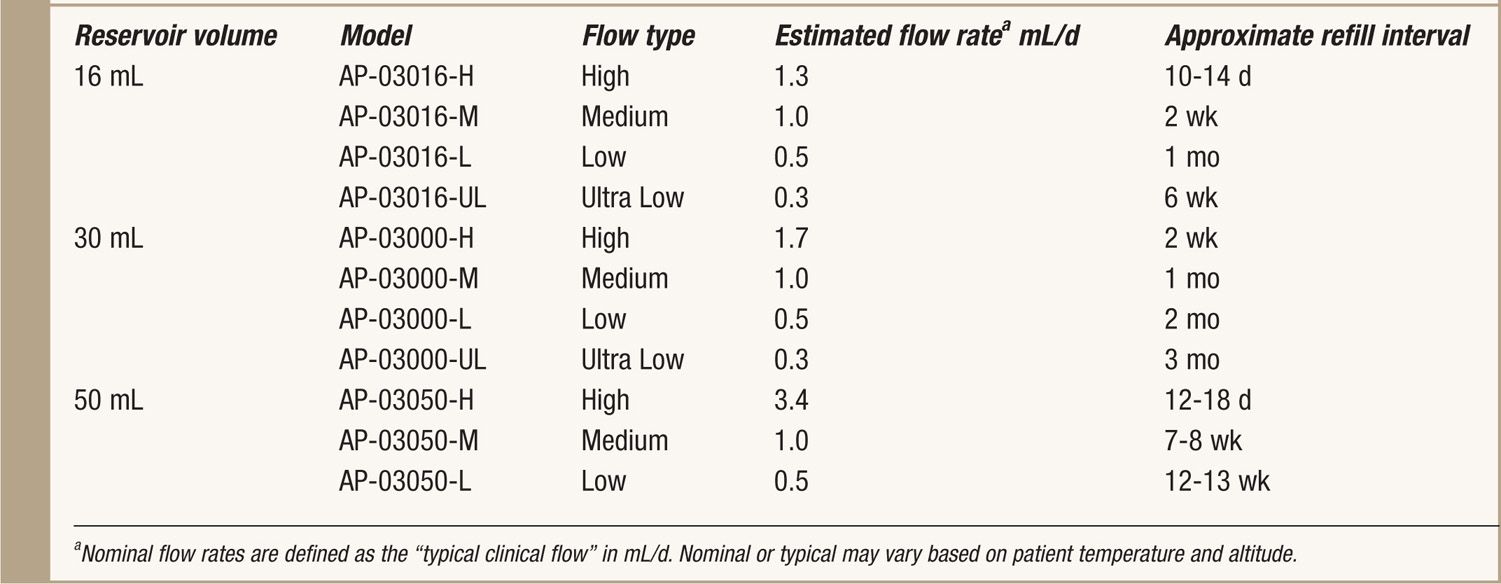
Design
• The Codman pump is a continuous infusion mechanical device (no battery or programmability) of the type described previously.
• It is available in 3 reservoir sizes: 16, 30, and 50 mL.
• The manufacturer supplies multiple flow rates for each reservoir size (Table 65-1).
• A novel feature of this pump is a bypass port for bolus dosing that allows direct access to the catheter through the same septum as the refill port (Figure 65-3).
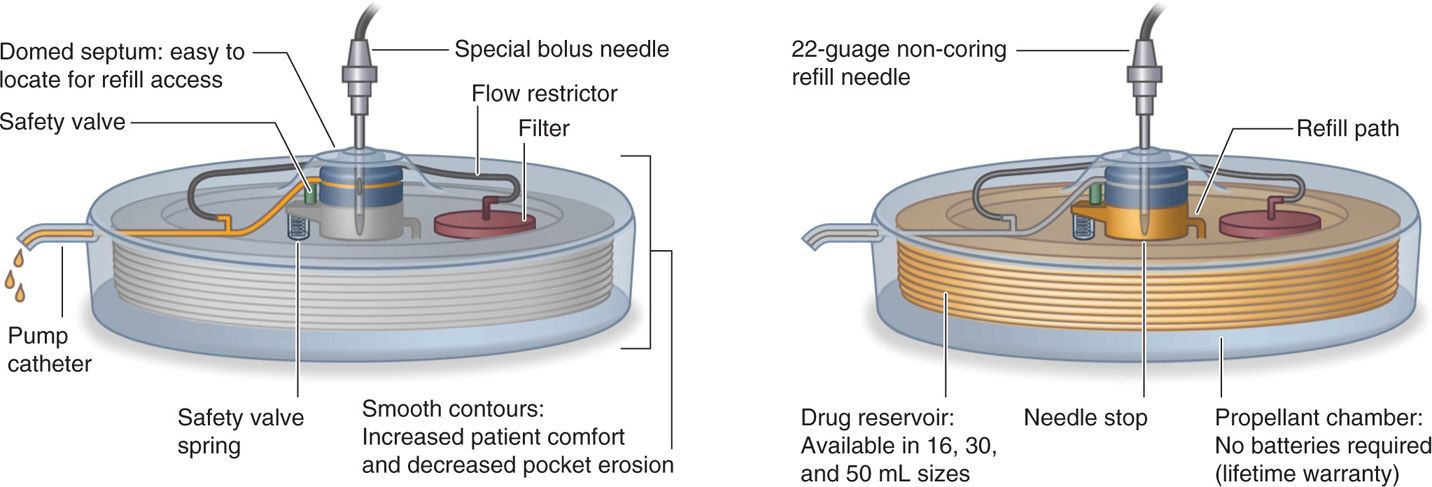
Figure 65-3. Schematic drawing Codman 3000: bolus injection demonstrated (left) and pump refill (right).
• A special, noncoring bolus needle is used and available from the manufacturer.
• It has a large raised domed port in the center of the pump for refill access.
MRI Compatibility
The manufacturer states: “The pump is made of surgical grade titanium which does not affect an MRI and has had extensive testing done by Codman to confirm this.”
PROGRAMMABLE PUMPS
Fundamentals
• The programmable pump also uses a propellant stored in an outside chamber to exert constant pressure on a bellows compartment that collapses as the drug is infused.
• Uses battery-powered electronics and motor to move the drug from the reservoir via a peristaltic pump through the intraspinal catheter.
• The pump delivers the prescribed medication in precise amounts based on the instructions that the clinician programs into the device.
• The device incorporates a catheter access port (CAP) that allows injection directly into the implanted catheter for drug administration and diagnostic purposes.
• The advantage of a programmable pump is that dose changes can occur simply by the use of an external programmer, flow rates are available over a much broader range, and the flow rates can be tailored so that the patient can receive different doses at selected times of the day.
• Clinical information is stored in the pump’s memory including recent programming changes, drug concentrations and flow rates, catheter length, battery life indication and refill dates. Current battery life is estimated at 6 to 7 years.
• New technology (myPTM Personal Therapy Manager, Medtronic, Inc, Minneapolis, MA) allows for patient activated boluses within the dosage and frequency prescribed by the managing physician. This may facilitate treatment of patients who experience intermittent pain of varying intensity.
Regardless of the manufacturer or design of the IDDS, patient safety is of paramount importance. Best practices recommendations and manufacturer’s instructions must be followed to avoid pocket refill, unintended direct catheter injection and untoward medication interactions. Appropriate training and familiarity with the device is essential.
A number of manufacturers have developed pumps, including programmable devices (eg, MedStream, Codman & Shurtleff, Inc, a Johnson & Johnson company, Raynham, MA, US and Prometra, InSet Technologies Incorporated, Mt. Olive, NJ, US) that are undergoing clinical trials or have received approval in Europe or elsewhere. The focus of this chapter will be to present technical descriptions of commercially available implantable intrathecal drug administration systems presently indicated for treatment of chronic pain in the United States—SynchroMed EL (10 and 18 mL) and SynchroMed II (20 and 40 mL) (Figure 65-4).
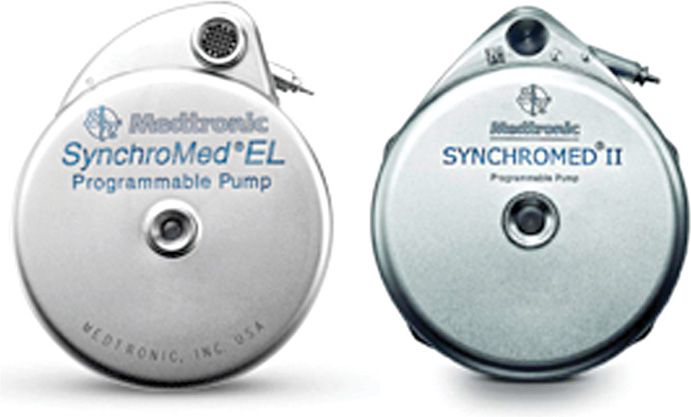
Figure 65-4. SynchroMed EL (left) and SynchroMed II (right).
SYNCHROMED EL BY MEDTRONIC
This model is no longer in production by Medtronic and is succeeded by their newer model, the SynchroMed II with the earlier model phased out after the initial FDA approval in 2004. However, as thousands of the SynchroMed EL pumps were implanted worldwide, many patients are still actively receiving therapy with this model. Therefore, design and clinical considerations for both pumps are included.
Indications
• Intrathecal (or epidural) infusion of preservative-free morphine for treatment of chronic pain. (Current labeling is for maximum concentration of 25 mg/mL.)
• Intrathecal infusion of preservative-free ziconotide (Prialt) for treatment of chronic pain. (Current labeling is for maximum concentration of 100 μg/mL.)
• Intrathecal infusion of baclofen (Lioresal) for treatment of spasticity.
• In addition to their application for intrathecal delivery, these pumps can be utilized for intravascular infusions of methotrexate, FUDR, doxorubicin, cisplatin, for cancer and clindamycin for osteomyelitis.
Drugs other than the FDA approved medications listed above are commonly used. However, the clinician should be aware that compatibility with other medications and mixtures of medications has not been well studied. Reports of corrosion, pump stall and other failures have been attributed to use of nonapproved medications.
Specifications
See Table 65-2.
TABLE 65-2. Specifics of SynchroMed EL
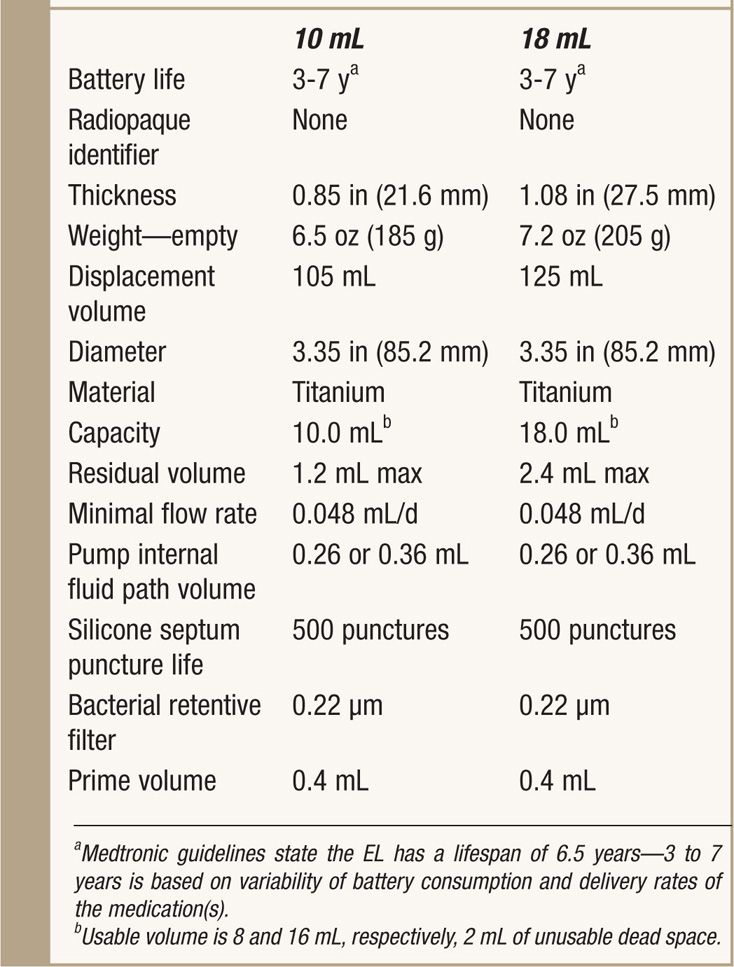
Design
• 3 sealed chambers:
1. Reservoir propellant
2. Battery and electronic module
3. Peristaltic pump and drug reservoir
• The peristaltic pump is what drives the medication from the pump into the catheter and eventually out of the tip into the intrathecal space. It is a two-roller model, which also acts as a valve that prevents introduction of fluid into the reservoir when capacity is reached.
• Side catheter access port—access with a 25-gauge needle for either drug administration or diagnostic purposes. There is a mesh screen covering the port; however, an external bacterial filter on the syringe is required when injecting into the port. Any introduction of fluid through this port will flow strictly forward through the system and out of the catheter. Therefore, one could inject radiopaque dye through the port to perform a myelogram without fear of introducing dye into the reservoir and distorting the concentrations of the drugs.
• Stability of morphine and baclofen has been tested and approved to 90 days with the SynchroMed EL (Figures 65-5 and 65-6).
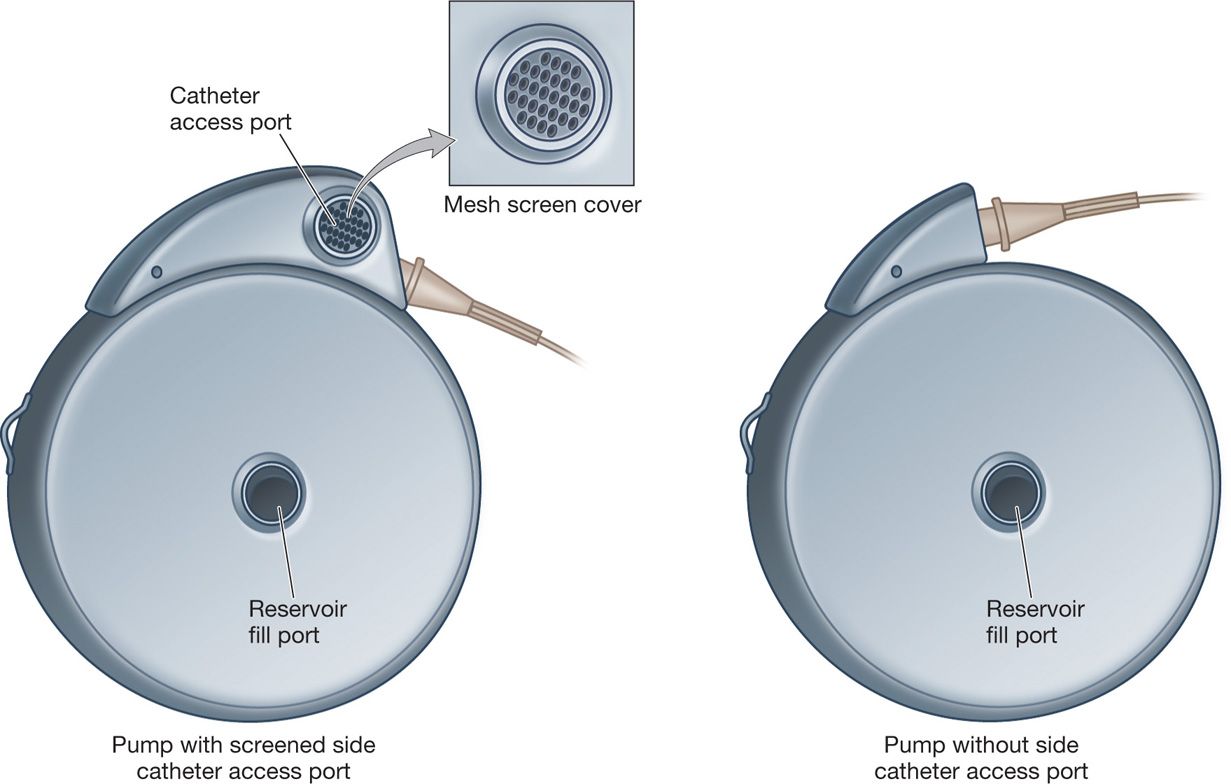
Figure 65-5. SynchroMed EL.
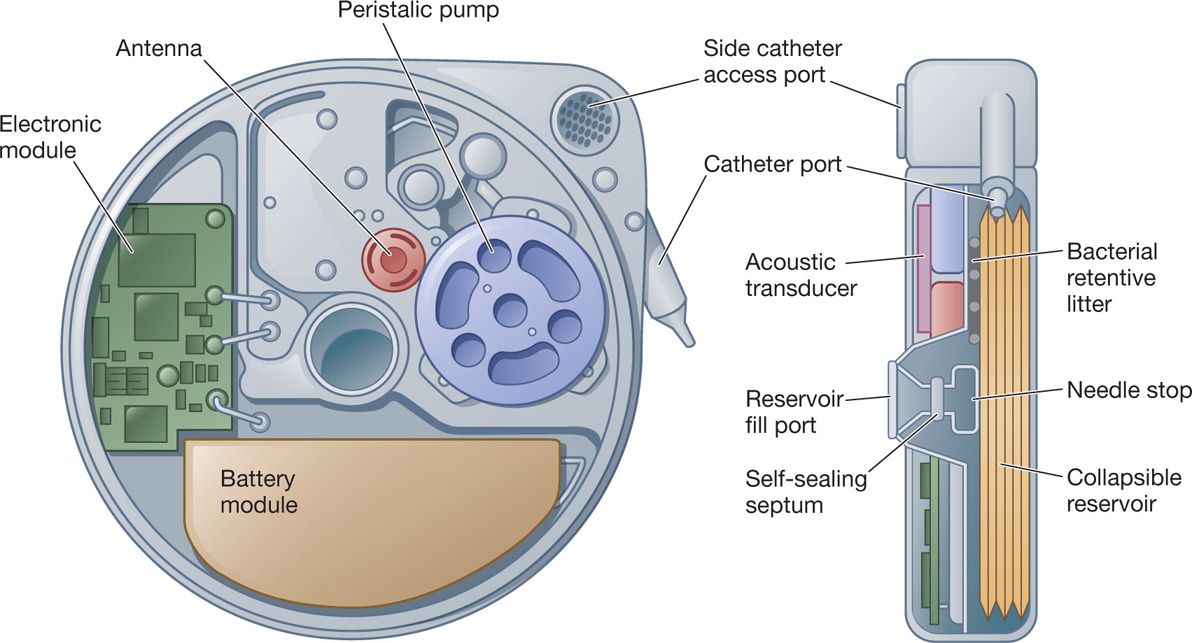
Figure 65-6. Schematic drawing of the pump.
Precautions During Implantation
• Pump needs to be warmed to body temperature prior to implantation to ensure proper function. If the SynchroMed EL is not warmed prior to voiding of the reservoir and initial filling, incomplete emptying can occur, which can lead to possible overfill of the reservoir and subsequent activation of the reservoir valve.
• Since the SynchroMed EL pump did not detect if the motor was stalled (unlike the newer SynchroMed II pump) a “pump purge” was recommended to ensure that the pump was functional.
• Implantation of the pump requires a maximum recommended depth of 1 in (2.5 cm) from the skin surface. Greater depth can cause difficulty with programming as well as difficulties with refilling as it can inhibit access to the pump’s septum during refilling.
Initial Programming
• The SynchroMed EL has 6 settings for drug administration:
1. Single bolus, which is programmed in drug units, ie, μg, mg, etc, over a predetermined time.
2. Priming bolus, which is programmed in volume (mL) over a predetermined time.
3. Bridge bolus, which is programmed when changing concentrations to deliver the old medication in the catheter and pump tubing at one flow rate and the new medication at a different flow rate.
4. Continuous infusion at a predefined rate.
5. Complex continuous infusion administers the reservoir contents at multiple rates. Between 2 and 10 variations are possible, with each variation programmed at its own individual predefined rate.
6. Periodic bolus for a determined period at specific intervals.
• When using the periodic bolus setting, the pump must be set for a flow rate of at least 1.8 μL/h.
• The internal computer is also capable of storing information specific to the pump being implanted (ie, model number and reservoir size) as well as the individual patient (ie, drugs present, concentration, rates, catheter length, etc). However, due to limits in memory—patient identification is limited to 3 characters and drug name to 5.
• The alarm must be programmed at the initial implant for low reservoir volume.
• If using the red “Therapy Stop” button on the programmer to stop the pump, it will not obtain or provide pump status information, and the pump will decrease its infusion rate to 0 μL/d.
MRI Compatibility
The SynchroMed EL is approved for MRI scanning. Pump performance has not been established for greater than 3.0 Tesla (T) horizontal, closed-bore MRI scanners.
• The magnetic field of the MRI scanner will temporarily stop the rotor of the SynchroMed EL pump motor and suspend drug infusion for the duration of the MRI exposure.
• The pump should resume normal operation upon termination of MRI exposure; however, there is the potential for an extended delay in pump recovery after exiting the MRI magnetic field because exposure to the MRI magnetic field may cause the motor gears within the pump to bind temporarily without permanent damage.
• This temporary binding may delay the return of proper infusion after the pump is removed from the MRI magnetic field.
• While extended delays in pump recovery are unlikely, reports have indicated that there is the potential for a 2- to 24-hour delay in return to proper drug infusion after completion of an MRI scan.
• Instructions for MRI safety are provided by the manufacture.
SYNCHROMED II BY MEDTRONIC
Indications
The SynchroMed II has similar indications as its predecessor (SMII is not indicated for doxorubicin, cisplatin, or clindamycin). It was created as an updated version of the EL and meant to improve upon the ease of use and allow more options for increased customization (Figure 65-7).
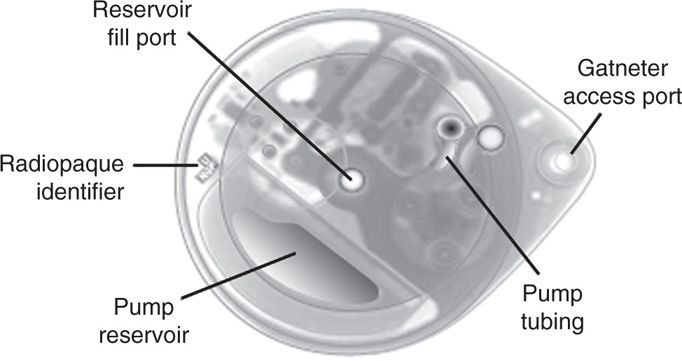
Figure 65-7. SynchroMed II.
Specifications
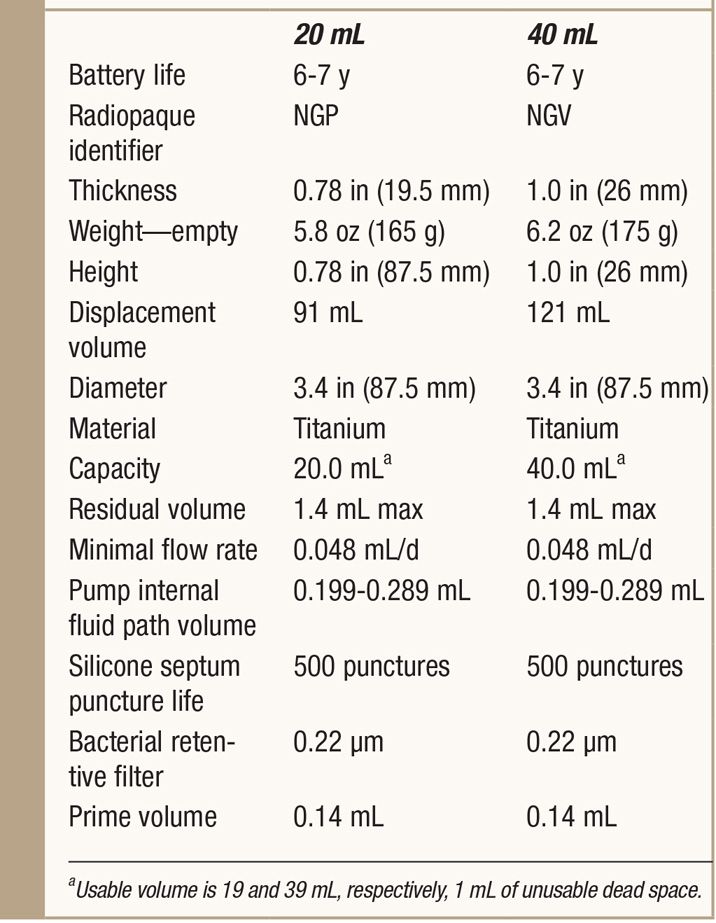
See Table 65-3.
TABLE 65-3. Specifics of SynchroMed II
Design
The essential design is relatively similar to the SynchroMed EL, with several differences highlighted below:
• Improved form factor
• 3 roller system with radiopaque ball marker on one of the roller arms for identification
• Refined mechanics and electronics allow for more precise infusion of medication as compared to the predecessor
• Pressure-activated reservoir (compared to the volume activated valve in the EL). As the reservoir fills, a pressure diaphragm flattens resulting in a spring-driven valve stem to form a seal—this allows for immediate release of the seal with aspiration (EL requires continuous aspiration and time for valve release).
• Larger catheter access port allowing for as large as a 24-gauge needle as well as a funnel design for easier access.
Precautions During Implantation
• Does not require warming or a pump purge prior to implantation.
• Implantation of the pump requires a maximum depth of 1 in (2.5 cm) from the skin surface. Greater depth can cause difficulty with programming as well as difficulties with refilling as it can inhibit access to the pump’s septum during refilling.
Initial Programming
• The SynchroMed II has settings which are similar to the EL with new features that allow further customization of dosing regimens:
1. Single bolus over a predetermined time.
2. Continuous infusion at a predefined rate.
3. Flex infusion which is similar to the complex continuous infusion of the EL. However, this version allows up to 13 different sequences to be programmed, each with independent rates and durations. In addition, a “basal rate” is programmed that runs whenever another sequence is not programmed. If desired, two “patterns” can be programmed to deliver different dosing for two sets of consecutive days throughout the week.
4. Minimum rate infusion—the minimum rate is 6 μL/d (0.25 μL/h)
5. Predetermined bolus—single bolus, priming bolus, or bridge bolus
6. Stopped pump mode—can be used for periods of up to 48 hours before an alarm will sound and potential damage to the pump may occur.
• SynchroMed II can store a patient’s full name and drug names up to 25 characters.
• Individual drugs, their concentrations, and even physician notes for communication with other clinicians are all possible.
• Programming/interrogation takes somewhat longer due to the increased amount of information stored within the pump as compared to the EL.
• One tone alarm for noncritical alert (ie, low reservoir volumes, elective replacement indicator, and a noncritical pump memory error).
• Two tone alarm for critical alert (ie, an empty reservoir, end of service, motor stall, stopped pump duration greater than 48 hours, and a critical pump memory error).
• When stopping the pump with the “stop key,” the SynchroMed II pump will provide the pump status just before shutting down and will also program the pump to the minimum infusion rate of 6 μL/d so as to avoid pump damage that can occur if the pump is left completely stopped for an extended period of time.
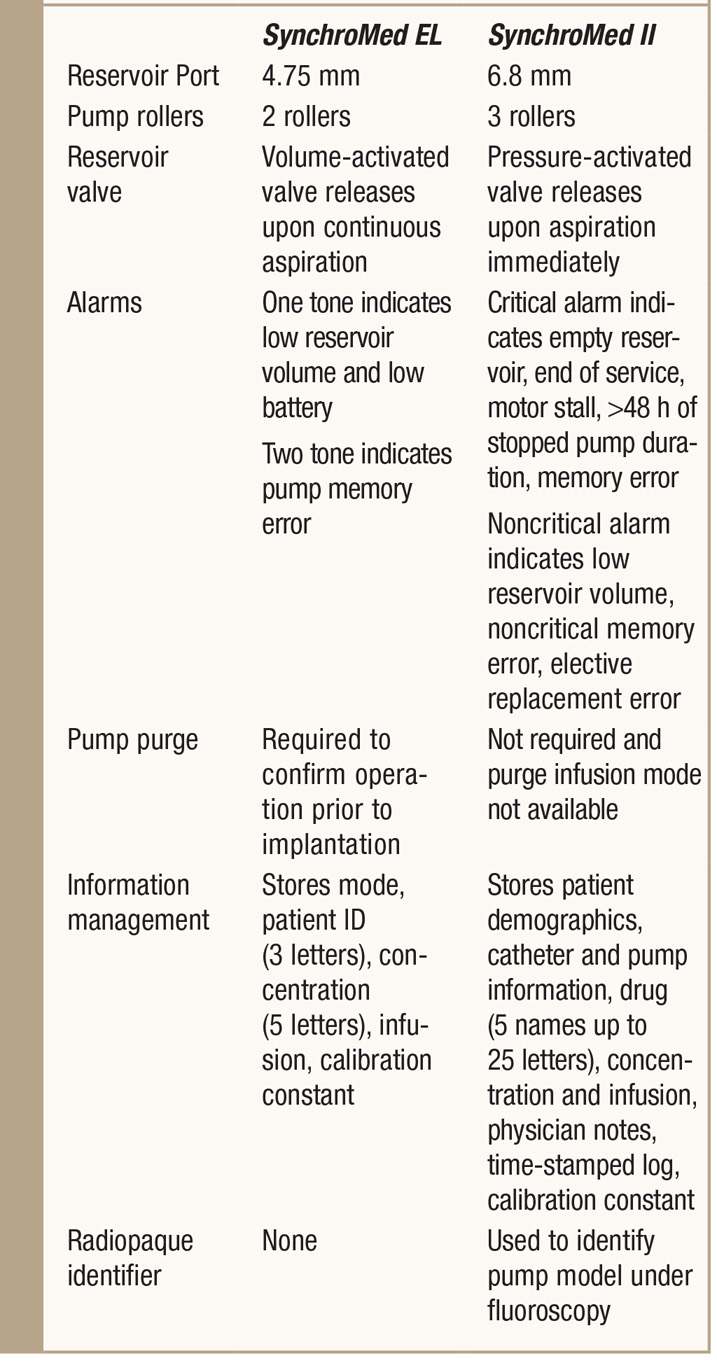
TABLE 65-4. Direct comparison of the SynchroMed EL to the SynchroMed II
MRI Compatibility
SynchroMed II compatibility has been established for use with up to 3.0 Tesla (T) horizontal, closed-bore MRI scanners. SynchroMed II pump performance has not been established with magnetic fields higher than 3.0 (T).
• The magnetic field of the MRI scanner will temporarily stop the rotor of the SynchroMed II pump motor and suspend drug infusion for the duration of the MRI exposure.
• The pump should resume normal operation upon termination of MRI exposure; however, there is the potential for an extended delay in pump recovery after exiting the MRI magnetic field because exposure to the MRI magnetic field may cause the motor gears within the pump to bind temporarily without permanent damage.
• While extended delays in pump recovery are unlikely, reports have indicated that there is the potential for a 2- to 24-hour delay in return to proper drug infusion after completion of an MRI scan.
• The SynchroMed II pump detects motor stall and motor stall recovery.
• Medtronic does not recommend programming the SynchroMed II pump to “stopped pump mode” prior to an MRI because of the possibility of an increased delay in the detection of an extended motor stall.
• Motor stall events are recorded in the pump event log and can be reviewed using the clinician programmer.
• A motor stall will also cause the pump alarm to sound (2-tone alarm). Stall recovery detection should occur within 20 minutes of exiting the MRI magnetic field. The detection of a motor stall and detection of motor stall recovery may each take up to 90 minutes if the pump is programmed to minimum rate mode (0.006 mL/d).
POTENTIAL COMPLICATIONS AND PITFALLS
Codman 3000
• Care should be taken not to use the special bolus needle for refilling the pump—as an inadvertent overdose is possible (IFU, Codman Refill Kit Model 3000 series REF AP-07014).
• Changes in body temperature or sea altitude can change the infusion rate, thus altering the refill interval.
SynchroMed EL
• Variable lifespan may need replacement as early as 3 years after implant due to battery depletion from higher infusion rates.
• Limited internal memory—can only store 3 characters of patient’s name and 5 of the drug.
• Erratic flow rates can occur at reservoir volumes below 2 mL with the potential for interruption of therapy.
• Prior to implantation, the pump needs to be warmed to body temperature and purged to ensure proper function.
SynchroMed II
• While the lifespan is much less variable than the EL, battery life can still be dependent on infusion rates.
• Once the reservoir volume drops below 1 mL, erratic flow rates can occur with the potential for interruption of therapy.
• Longer communication time required between interrogator and pump due to increased amount of information to be transferred.

Figure 65-8. Comparison of width between models.
For both devices, compounded drugs and admixtures of unapproved medications can cause the development of impurities within the pump resulting in damage and potential pump failure. An external filter on the syringe should be used when injecting into the catheter access port (side port). Pump function can be affected by radiation, lithotripsy or if the patient exposes themselves to elevated temperature or changes in pressure for extended periods of time.
CLINICAL PEARLS
Codman 3000
• Does not require an energy source or complex programming circuitry and therefore does not need replacement because of battery or a specialized programmer.
• Any dosage adjustment requires emptying and refilling the pump with a newly formulated drug concentration.
• Timed bolus dosing, varying flow rates, and on-board storage of clinical data are not possible.
Several important clinical changes occurred with the introduction of the SynchroMed II:
• Reservoir size increased from 10 and 18 mL to 20 and 40 mL. This allows for longer refill intervals, reduced costs, and improved patient satisfaction.
• Device size was reduced considerably: the SynchroMed El 18 mL pump has a displacement volume of 125 mL, while the SynchroMed II 20 mL pump displaces just 91 mL and the 40 mL reservoir pump displaces 121 mL. This improves cosmesis and patient acceptance.
• Morphine and baclofen have been tested and approved for stability in the reservoir for 180 days—doubling approved stability.
• Continued approval for intrathecal delivery of preservative-free morphine sulfate, preservative-free ziconotide (Prialt), and baclofen (Lioresal).
• Design consisting of a three roller pump system with a radiopaque ball marker on one of the roller arms (can track roller rotation for a “roller study” to detect pump stall).
• Improved accuracy of flow rates and improved memory capacity.
• Pressure diaphragm in reservoir—immediate aspiration from the reservoir.
• Newer infusion modes—flex infusion and minimum rate infusion mode.
• The myPTM device, compatible only with SynchroMed II, allows patients who experience episodes of intermittent pain to dispense a bolus dose of medication (within physician-prescribed limits) when needed.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






