2. Multifocal causes are the most diverse group and account for the largest cohort of cases of coma [4]. Trauma is the leading cause of coma followed by vascular lesions and anoxia. Of the postanoxic causes, the most common are cardiopulmonary arrest, stroke, respiratory arrest, and carbon monoxide poisoning. Other common causes include the postictal state after a seizure, intoxications, and metabolic derangements.

FIGURE 31.1 Components of consciousness.
CLINICAL PEARL
Traumatic brain injury remains the leading cause of coma. A Glasgow Coma Scale (GCS) of <8 at the time of admission remains an independent predictor of mortality.
C. Differential diagnosis of comatose states
3
1. The differential diagnosis of coma should involve ruling out other global disorders of consciousness including VS, locked-in syndrome, akinetic mutism, and catatonia [5].
2. VS is notable for preserved arousal mechanisms associated with a complete lack of self or environmental awareness. Hypothalamic and brainstem function persists; hence patients can survive in these states for prolonged intervals of time. Movements in response to external stimuli can occur but are reflexive and unreproducible. Patients can have spontaneous eye opening but do not track.
3. Akinetic mutism is defined as a state of profound apathy with preserved awareness, revealed by attentive visual pursuit, but a paucity and slowness of voluntary movements not due to paralysis. A distinguishing feature is that these patients do not have spasticity or abnormal reflexes.
4. Locked-in syndrome patients have a largely intact consciousness but a severely limited ability to communicate their awareness due to paralysis of voluntary muscle. It is associated with acute injury to the ventral pons, just below the level of the third nerve nuclei, thus classically sparing vertical eye movements and blinking [6].
5. Catatonia is a complication of psychiatric illnesses such as severe depression, bipolar disorder, or schizophrenia, in which patients have open eyes but do not speak or move spontaneously and do not follow commands, with an otherwise normal neurologic examination and an electroencephalogram showing low voltage but no slowing.
D. Approach and management of a comatose patient
1. Initial management
a. The clinical approach to a comatose patient is outlined in Figure 31.2. Once a patient is established as being unarousable and nonresponsive, the ABCs (airway, breathing, and circulation) are the most important first steps in care. Airway protection by intubation (indicated with a GCS ≤ 8) and circulatory/hemodynamic management are critical to limiting secondary insults.
(1) Mean arterial pressure (MAP) of less than 70 mm Hg should be treated with volume expansion and/or vasopressors.
(2) MAPs of greater than 130 mm Hg should be treated with antihypertensives.
(3) Initial laboratory tests should include a complete blood count, electrolyte panel with chemistries (i.e., magnesium, phosphorus, calcium, BUN, creatinine, lactate, and osmolarity), arterial blood gas, liver functions, PT and PTT, drug screen, and ECG.
(4) Emperically administer thiamine 100 mg IV in patients who appear malnourished, have strong alcohol abuse history, or have unexplained coma.
3 4 5
(5) Once vital signs are stabilized, a noncontrast head computed tomography (CT) should be done to assess for intracranial structural changes; for example, intracranial hemorrhage and subarachnoid hemorrhage, acute hydrocephalus, masses, and cerebral edema.
(6) If there are signs of a herniation syndrome, give 20% mannitol 1 g/kg IV bolus or hypertonic saline.
CLINICAL PEARL
Hyperventilation as a routine in the management of cerebral edema is no longer recommended. Normocarbia is the norm.
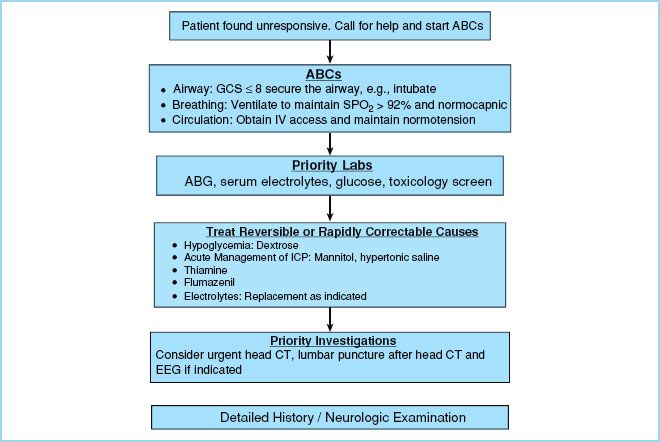
FIGURE 31.2 Clinical approach to a comatose patient.
2. Secondary evaluation and management
a. Once the patient is stabilized, a more thorough history and physical examination may be done to identify causes that are not already apparent and to identify concurrent pathologies. The history of present illness can be obtained from caregivers or family members, witnesses, the emergency medical technicians, clinical records, and medication history. Important elements include the time course, prior focal signs, prior neurologic events, and recent illnesses. Although a complete physical examination is warranted, this chapter will focus on a brief and directed neurologic examination.
b. By definition, the comatose patient is unarousable and unaware. This is confirmed by the presence or absence of eye opening (awareness) and the response to verbal and somatosensory stimulation (arousability). A noxious stimulus is used to assess arousability. Techniques include squeezing the trapezius, supraorbital pressure, sternal rub, nail bed, or temporomandibular pressure. Reflex responses (i.e., posturing) may be present and arise from subcortical structures.
c. In the comatose patient the most important cranial nerve reflexes are the pupillary, corneal, and vestibuloocular reflex. Pupil size, position, and reaction to light are individually assessed. Normal pupil size is 3 to 7 mm. A discrepancy of 1 mm in pupil size may be normal. Certain toxidromes are associated with either miosis or mydriasis. For example, the following toxidromes are associated with mydriasis: Sympathomimetic agents, anticholinergic agents, serotonin syndrome, and tricyclic antidepressants. Miosis is associated with opioid agents, sedative-hypnotic agents, and cholinergic agents.
d. An impaired pupillary response to light, either bilateral or unilateral, usually indicates a primary brainstem lesion or herniation.
3. Diagnostic testing of coma
a. CT scan is sensitive for detecting structural pathology that needs immediate intervention such as cerebral edema, tumor, hemorrhage, and herniation. It is less useful in acute ischemia and toxic metabolic syndromes.
CLINICAL PEARL
Cerebral edema is a key factor in the final common pathway in coma due to a number of causes including traumatic brain injury, carbon monoxide toxicity, hepatic encephalopathy, and uremic coma.
b. Magnetic resonance imaging (MRI) is usually warranted in comatose patients if the initial CT was normal or equivocal. MRI is more sensitive than CT for detecting diffuse axonal injury, acute ischemia, and venous sinus thrombosis [7].
c. Electroencephalography (EEG) is indicated in cases of unexplained coma. Nonconvulsive status epilepticus (NCSE) has been reported as high as 9% to 18% in patients with unexplained coma [8]. In patients without epilepsy, periodic epileptiform discharges (PEDs) are suggestive of underlying brain injury. In toxic and metabolic causes of coma, as patients progress from lethargy to coma, there is diffuse slowing of background EEG rhythms and changes from alpha to theta and, subsequently, delta activity on the EEG. Patients with hepatic disease, azotemia, and profound hypoglycemia can manifest blunt spike-and-slow wave complexes termed triphasic waves. Though not pathognomonic, they can be suggestive of a metabolic encephalopathy [9]. But the greatest value is in differentiating ictal encephalopathies from psychiatric disease as etiology of coma.
CLINICAL PEARL
NCSE is often an underdiagnosed condition. Patients on IV sedation in the ICU including propofol can still have NCSE. Continuous EEG is valuable in establishing the diagnosis.
d. Lumbar puncture (LP): LP should be considered in suspected infectious and inflammatory causes of coma. However, one must exercise caution that there are no clinical signs of “impending” herniation, and that the CT is negative for potential herniation syndromes before proceeding with the LP [10,11].
4. Assessment of coma: The coma scales
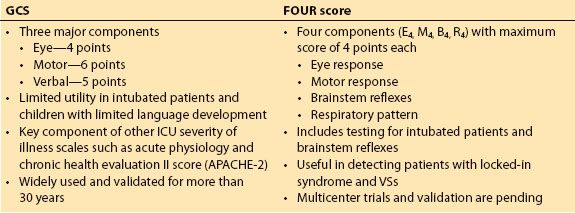
a. The GCS was initially devised for patients with traumatic brain injury [12], but has gained widespread acceptance and stood the test of time over the past 3 decades as a bedside tool for evaluating the level of consciousness in virtually all acutely ill patients [13]. The Glasgow scale was originally developed using simple parameters for the specific purpose of allowing less experienced doctors and other health professionals to produce an accurate report of a patient’s state of consciousness. The Glasgow scale is a composite sum obtained by assessing the following three parameters: Eye opening, best verbal response, and best motor response. The score varies between 3 and 15 points [14] (see chapter 29).
b. Major limitations of GCS are its inability to accurately assess intubated patients, difficulty in assessing aphasic patients, and limited utility in children, particularly those less than 3 years of age and prior to acquisition of language [15].
c. Attempts have been made to modify the GCS; however, most of these scales were more complicated and were seldom used outside their countries of origin. Similarly, other scales (e.g., Innsbruck coma scale, reaction level scale (RLS85), FOUR score) have been developed since, yet none have gained similar widespread acceptance like the GCS. The FOUR score was described in 2005 and is composed of four elements (eye response, motor response, brainstem response, and respiration); each with a maximum score of four [16]. It was designed to overcome the shortcomings of the GCS by being able to evaluate intubated patients and evaluate brainstem function. However, it is yet to be validated across multiple centers [15]. A comparison of the GCS and FOUR score are presented in Table 31.2.
Table 31.2 Comparing glasgow coma scale and FOUR score coma scale
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







