Route
Advantages
Disadvantages
Intravenous
Can be titrated and delivered continuously
Requires IV access, may have more side effects, may progress to anesthesia more easily
Oral
Simple, often most acceptable method to child, wide choice of drugs
Needs co-operation of child to take medication, slow onset, not titratable
Intranasal
Rapid onset, can be repeated
Unpleasant for the child, only effective for certain drugs
Intramuscular
Relatively rapid onset, better for uncooperative children
Painful, not titratable, absorption may be delayed in shock
Inhalational
Rapid onset and offset, readily titratable
Needs specialized drugs and equipment, may be unacceptable to younger children
Rectal
Very effective for certain drugs (barbiturates, chloral hydrate)
Slow onset, may not be acceptable to the child or parents
By far the commonest method used is by mouth, as this is often the most acceptable to the child and parents. However, intravenous administration offers the advantages of very rapid onset, titratability and repeatability, the option of continuous infusion and the possibility of fewer peaks and troughs in sedation. The main disadvantage is the need for vascular access, which may be challenging, particularly in the younger child. For the purposes of this chapter, the focus will be on intravenous sedation.
Measuring Level of Sedation
There are a number of different ways of assessing the level of sedation in patients. The simplest way is to make a clinical assessment based on the degree of hypnosis the patient exhibits and their response to verbal and tactile stimuli.
Alternative methods include more complex sedation scoring systems, which are mainly for situations of prolonged sedation such as on PICU, or when a less subjective method of assessment is necessary, such as during drug trials .
The primary purpose of a sedation scale is to provide a repeatable, objective assessment of the level of sedation. This allows the operator to recognize when the patient is becoming over-sedated and to avoid this from happening, thereby pre-empting serious complications.
Sedation Scoring Systems
A number of different scoring systems have been developed for both adults and children to assess the depth of sedation. In reality, the majority of patients do not need to have the depth of sedation scored, as a simple clinical assessment is sufficient. However, for documentation purposes, or to guide interventions, it may be useful to use a more defined assessment. The main disadvantage of any scoring system is that deeper levels of sedation inevitably require a degree of stimulation of the patient for assessment purposes, which may be counterproductive, negating the benefits of sedating the patient in the first place. Any scale or system that is used should be simple, consistent, and reproducible, both between observers and from episode to episode.
Scoring systems or scales can be divided into general scales/scores that can be used in both adults and children and specific pediatric scales/scores.
General Scoring Systems/Scales
Perhaps the two most commonly used sedation-scoring systems are the Ramsey scale and the Aldrete score.
Ramsay Scale
The Ramsay Sedation scale was the first scoring system to be used for sedated patients. It was developed in 1974 as an objective assessment of sedation in adult intensive care patients receiving an alphaxalone-alphadalone (Althesin) infusion [4]. It is intuitive, easy to remember, and can be used in any situation where patients are sedated, including PICU, procedural sedation, and imaging. Scores 1–3 apply to patients who are awake , whilst 4–6 apply to those who are asleep (Table 26.2).
Table 26.2
Ramsay scale for sedation
Sedation score | Clinical response |
|---|---|
1 | Fully awake |
2 | Drowsy but awakens spontaneously |
3 | Asleep but arouses and responds appropriately to simple verbal commands |
4 | Asleep, unresponsive to commands, but arouses to glabellar tap or loud verbal stimulus |
5 | Asleep and only responds to light glabellar tap and loud verbal stimulus |
6 | Asleep and unresponsive to light glabellar tap and loud verbal stimulus |
Aldrete Score
The Aldrete score was introduced into practice in 1970, as a postsurgical equivalent of the Apgar score [5]. Like the Apgar score, it contains 5 parameters to assess, and the total score can range from 0 to 10. Although it can be used as an assessment of the level of sedation, it was actually created for use in patients recovering from anesthesia. Patients are seldom significantly hypotensive, apneic, unresponsive and cyanotic, after sedation, and therefore this score has limited usefulness in this group, other than as an assessment of the level of recovery following treatment (Table 26.3).
Table 26.3
Aldrete sedation scale
Activity | |
Voluntary movement of all limbs to command | 2 points |
Voluntary movement of 2 extremities to command | 1 point |
Unable to move | 0 points |
Respiration | |
Breathe deeply and cough | 2 points |
Dyspnea, hypoventilation | 1 point |
Apneic | 0 points |
Circulation | |
BP ± 20 mmHg of preanesthesia level | 2 points |
BP ± 20–50 mmHg of preanesthesia level | 1 point |
BP ± 50 mmHg of preanesthesia level | 0 points |
Consciousness | |
Fully awake | 2 points |
Arousable | 1 point |
Unresponsive | 0 points |
Color | |
Pink | 2 points |
Pale, blotchy | 1 point |
Cyanotic | 0 points |
The Observer’s Assessment of Alertness/Sedation
The OAAS scale was originally developed in the 1990s to assess the level of sedation with midazolam in adults [6]. It used assessments of responsiveness, speech, facial expression, and eyes to produce a score between 1 and 5. Unlike the Ramsey scale, the OAAS score reduces as the level of sedation increases (Table 26.4).
Table 26.4
Original Observer’s Assessment of Alertness/Sedation scale
Responsiveness | Speech | Facial Expression | Eyes | Score |
|---|---|---|---|---|
Responds readily to name spoken in normal tone | Normal | Normal | Clear, no ptosis | 5 |
Lethargic response to name spoken in normal tone | Mild slowing | Mild relaxation | Glazed or mild ptosis (<half the eye) | 4 |
Responds only after name is called out loudly and/or repeatedly | Slurring or prominent slowing | Marked relaxation | Glazed and marked ptosis | 3 |
Responds only after mild prodding or shaking | Few recognizable words | Marked relaxation | Glazed and marked ptosis | 2 |
Does not response to mild prodding or shaking | Few recognizable words | Marked relaxation | Glazed and marked ptosis | 1 |
The scale has subsequently been modified to make it simpler to use. In the modified version only the “responsiveness” category has been retained (Table 26.5).
Table 26.5
Modified Observer’s Assessment of Alertness/Sedation scale
Responds readily to name spoken in normal tone | 5 |
Lethargic response to name spoken in normal tone | 4 |
Responds only after name is called loudly and/or repeatedly | 3 |
Responds only after mild prodding or shaking | 2 |
Does not respond to mild prodding or shaking | 1 |
As with other adult sedation scales, it has never been validated in the pediatric population.
Pediatric Scoring Systems
A number of sedation scales specific to pediatric patients have been developed over the years, for both research and clinical use.
University of Michigan Sedation Scale
This is a very simple scoring system , which has been shown to be reliable and valid for assessing depth of sedation in children [7]. It is similar to the Ramsay score, but based on a 5-point, rather than a 6-point scale (Table 26.6).
Table 26.6
University of Michigan Sedation scale
0 | Awake and alert |
1 | Minimally sedated: Tired, sleepy, appropriate response to verbal conversation and/or sound |
2 | Moderately sedated: somnolent,/sleeping, easily aroused with light tactile stimulation or a simple verbal command |
3 | Deeply sedated: deep sleep, arousable with only significant physical stimulation |
4 | Unarousable |
COMFORT Scale
The COMFORT scale was designed primarily for use in PICU to assess the depth of sedation in non-paralyzed patients undergoing mechanical ventilation [8]. It relies on assessment of a number of behavioral and physiological parameters. One of the limitations is that physiological parameters can be adversely influenced by critical illness or the use of sedative drugs, making the score less accurate when this is the case. Interestingly, validation studies have suggested that these are the source of greatest interindividual variability during assessment, rather than the behavioral parameters. Removal of the physiological variables from a subsequent version of the scale (COMFORT-behavior or B) has improved the accuracy [9, 10]. It requires assessment of seven different variables, level of consciousness, calmness/agitation, respiratory response, crying, physical movement, muscular tone and facial tension, on a scale of 1 to 5. The main limitation is that the respiratory assessment is designed for patients undergoing mechanical ventilation. Another limitation is that it may also be less useful in distinguishing distress due to pain from distress due to other factors, such as delirium or drug withdrawal.
Faces Legs Activity Cry Consolability Scale (FLACC)
There is potentially a significant overlap between assessment of sedation and pain depending on the procedure. There are several scales that specifically relate to the assessment of pain. One of these is FLACC, which stands for Facial expression, Leg movement, Activity, Cry and Consolability. It was developed for use in younger children, due to the inability to report in preverbal infants and the lack of reliability of self-reporting in preschool children [11]. The five categories score between 0 (no pain) and 2 (severe pain) in each, giving a maximum score of 10 (Table 26.7).
Table 26.7
Faces, Legs, Activity, Cry Consolability (FLACC) scale
Categories | Scoring | ||
|---|---|---|---|
0 | 1 | 2 | |
Face | No particular expression or smile; disinterested | Occasional grimace or frown, withdrawn | Frequent to constant frown, quivering chin, clenched jaw |
Legs | Normal position or relaxed | Uneasy, restless, tense | Kicking or legs drawn up |
Activity | Lying quietly, normal position, moves easily | Squirming, shifting back and forth, tense | Arched, rigid or jerking |
Cry | No cry (awake or asleep) | Moans or whimpers; occasional complaint | Crying steadily, screams or sobs, frequent complaints |
Consolability | Content, relaxed | Reassured by occasional touching, hugging or being talked to; distractible | Difficult to console or comfort |
Clearly, it would be inappropriate to use a pure pain scale to assess level of sedation in the majority of patients, as untreated pain in any child is likely to produce both increased conscious level and movement; two situations we are trying to avoid by sedating the child in the first place. However, there may be some value in using FLACC during procedures where there is a significant painful stimulus, so that the analgesic component of the sedation is optimized .
Another commonly used pain scale is the Children’s Hospital of Philadelphia pain scale (CHEOPS) , which was developed in 1985 [12].
As previously mentioned, a big disadvantage of many sedation scales is that require some degree of stimulation of the patient in order to make the assessment. This is counterintuitive, as one of the ways to ensure success with sedation is to minimize the degree of patient stimulation, be it movement, noise, pain or something as simple as a blood pressure cuff inflating and deflating. By regularly disturbing the patient in order to make an assessment of depth of sedation, the observer is likely to guarantee failure of the technique. Physiological data is useful, but safe parameters for different ages of patient and levels of sedation have never been determined, so interventions can only be driven largely by protocol or experience. Clearly, parameters such as oxygen saturation, heart rate, blood pressure, respiratory rate, and end-tidal carbon dioxide may all give an indication of when sedation has become excessive and complications are imminent, but in an ideal world it should be possible to pre-empt that by responding to changes in physiology as it happens.
Other considerations when using sedation scales are that there is no consensus amongst users as to which is the best, measured parameters may vary significantly between scales, validation may be limited to a small number of studies, and performance reliability may differ significantly between individuals.
Depth of Sedation Monitoring
An alternative to using a sedation scale or score is to use one of a number of the commercially available depth of anesthesia (DOA) monitors . These have been used in children to assess the level of sedation in several different environments, including the Operating Theater, PICU, and the ED. By far the most commonly studied monitor is the Bispectral Index (BIS) . Several studies have used BIS monitoring to guide the administration of sedation [13–17]. However, studies in PICU patients comparing BIS with sedation scores such as Ramsay and COMFORT demonstrate moderate correlation only, with significant variation between patients [18–21].
Perhaps the best use of BIS is in distinguishing lighter levels of sedation from deeper levels in non-paralyzed patients, and for avoiding the patient lapsing into a state of general anesthesia, which has been described. In a study where propofol alone was administered for intrathecal chemotherapy and bone marrow aspirate, by a clinician blinded to the BIS value, the mean lowest BIS score was 29, with several patients achieving values of less than 20 [22]. Perhaps this was more a reflection of the inadequacy of the technique than of those who were using it, as there was no co-administration of an analgesic agent for what were stimulating interventions. A subsequent study demonstrated that a targeted BIS value of 45 was needed to provide adequate depth of sedation with propofol alone in children undergoing a range of painful procedures on PICU [15]. The mean dose of propofol administered to achieve this was more than 0.5 mg/kg/min (30 mg/kg/h). Given that the BIS range for general anesthesia is between 40 and 60, clearly most of these children were receiving anesthesia rather than deep sedation. A recent blinded study in the Emergency Department confirmed that nearly 80 % of patients were receiving general anesthesia rather than sedation, with a mean lowest BIS score of 43 [23]. Over 90 % of the sedating physicians underestimated the level of sedation.
As with assessments of depth of sedation, application of a monitor such as BIS may be quite stimulating for the patient. Ideally, the electrode should be attached before the start of sedative administration, so that the effect of stimulating the patient is minimized . However, some children will find this distressing, so that a balance needs to be found and will depend on the age of the child, the sedation being administered and the procedure being undertaken.
An alternative to the BIS monitor is the Narcotrend. The Narcotrend Index has been shown to correlate well with the depth of sedation in children undergoing upper GI endoscopy [24]. It also has the advantage that the sensor application is relatively non-stimulating and well tolerated by most children. Other systems that appear to correlate well with objective assessment of sedation are the Cerebral State Index and the A-line ARX [25].
The only hypnotic agent that does not show a good correlation between agent concentration and the output of the DOA monitors is ketamine, due to its excitatory effects on the EEG [17, 26]. There is also good evidence that in younger patients the BIS may be less accurate than in older children, due to differences in the underlying EEG pattern and it is certainly not indicated in patients less than 1 year of age [27].
Sedative Agents
A number of different drugs are currently used for sedation in children, either as a single agent or in combination with other drugs. The advantages of a single agent are that its effects are more predictable and controllable, and side effects are limited to those of one agent. Drug combinations can have the advantage of synergism, so that much lower doses of each agent can be used for a given effect. They may also combine effects, such as hypnosis, analgesia, and anxiolysis that no single agent can produce. The main disadvantages of combinations are the unpredictable response, increased risk of side effects, and potential for drug interactions. Table 26.8 lists the most commonly used agents for intravenous sedation, along with their advantages, disadvantages and the other drugs they are often used in combination with.
Table 26.8
Uses, advantages, and disadvantages of some of the most commonly used drugs in children
Drug | Class | Uses | Advs | Disadvs | Combination |
|---|---|---|---|---|---|
Propofol | Phenol derivative | Induction anesthesia Procedural sedation | Short half-life Can be administered as mass rate infusion or TCI | Painful to inject No analgesic properties Can easily cause anesthesia effects | Opioids Ketamine Midazolam Alpha 2 agonists |
Midazolam | Benzodiazepine | Procedural sedation | Anxiolysis Amnesia Can be antagonized | Some respiratory and CVS depression | Propofol Ketamine |
Ketamine | Phencyclidine derivative | Procedural sedation | Immobilization Analgesia CVS stability | ‘Dissociative’ state making sedation end points difficult to assess Hypersalivation Emergence phenomena Tachycardia & hypertension | Propofol Midazolam |
Dexmedetomidine | Alpha 2 agonist | Procedural sedation | Airway and ventilation maintained Immobilization Reduced analgesia requirements | Hypotension Bradycardia Relatively long half-life | Propofol |
Fentanyl | Synthetic opioid | Analgesia | Rapid onset Short duration Easily titratable CVS stability | Respiratory depression | Any hypnotic |
Single Agents
Propofol
Propofol or 2,6 di-isopropylphenol was first introduced into clinical practice in the late 1980s. It was marketed as having a faster recovery than thiopentone and less histamine release and chance of an allergic reaction. Propofol is formulated in soya and egg lecithin, but it can be safely used in soya and egg allergy patients, as they are allergic to the protein component of soya and egg, rather than the fat component. Patients in whom propofol should be avoided include those with mitochondrial disease, hereditary channelopathies, and acyl co-enzyme A dehydrogenase deficiency (MCADD and LCADD). The main side effects following administration are a dose-dependent reduction in respiratory rate, progressing to apnea, reduction in heart rate, vascular resistance and blood pressure, and extrapyramidal movements. Both onset and recovery are rapid; the latter due to redistribution. The half-life is about 30–60 min, making it an ideal agent for short-term sedation .
Mode of Administration
There are three main options when it comes to administering intravenous sedation to children. These are bolus dose, continuous intravenous infusion, or target-controlled infusion (TCI).
Bolus
The simplest method of administering propofol is by bolus injection. This has the advantage of not requiring any specific equipment. It is easy to titrate and repeated doses can be administered as required. The main disadvantage of bolus administration is that it leads to peaks and troughs in plasma concentration. This can exaggerate the side effects of propofol , such as respiratory depression and hypotension, although these will be short-lived. Also, the duration of sedation will be relatively short, requiring additional boluses. It is best for quick procedures, where variations in the depth of sedation are less important. An initial bolus of 0.5–1 mg/kg will produce an effect within 30 s and can be repeated as necessary. Larger doses (2–3 mg/kg) are likely to induce general anesthesia and almost invariably produce a period of apnea.
Clinical Example
A 6 m-old, 7.6 kg child, post-cardiac surgery, is scheduled for removal of a mediastinal chest drain. He is self-ventilating in wafting oxygen and analgesia is being provided by NCA morphine. A 20 mcg/kg bolus of morphine is administered from the NCA. After about 5 min, a propofol bolus of 5 mg (0.66 mg/kg) is given. The patient becomes only slightly sedated, so the bolus is repeated at the same dose. His conscious level reduces sufficiently for the drain to be removed successfully and the patient recovers quickly from the procedure.
The TivaTrainer© simulation in Fig. 26.1 demonstrates what happens to the plasma (red line) and effect-site (green line) concentration of propofol following the administration of the two boluses. The initial bolus leads to an effect-site concentration of just under 1 mcg/ml, which clearly isn’t enough to produce significant sedation. The second bolus has a cumulative effect, resulting in a rapid rise in the effect-site concentration to about 1.5 mcg/ml. In combination with the morphine, this is sufficient for the patient to become lightly sedated, with minimal effect on HR, BP, RR, or oxygen saturations. As the procedure is likely to be painful, administration of propofol alone would not have been appropriate and co-administration of an opioid is necessary. Care must be taken to ensure that the synergistic effect between propofol and opioids is considered when the drugs are given together. Patient movement would not have influenced the success of the procedure; so lighter levels of sedation can be tolerated and are advisable anyway in this case, given the underlying cardiac condition.
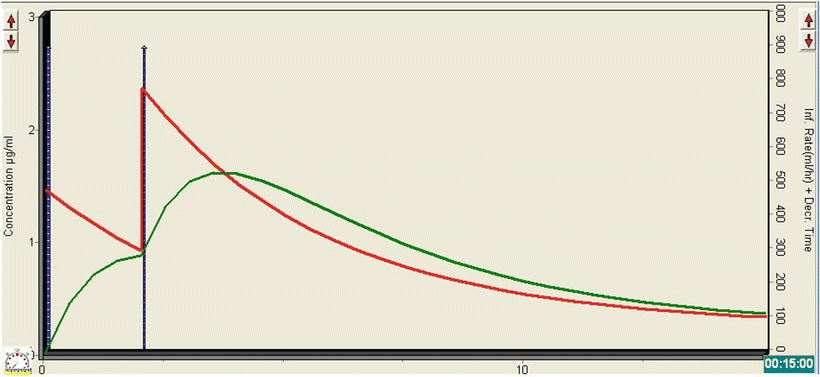

Fig. 26.1
TivaTrainer© simulation demonstrating the changes in plasma and effect-site concentration of propofol with the administration two bolus doses
Continuous Infusion
For longer procedures , where repeated bolus doses might be required, it makes more sense to administer propofol as a continuous infusion, preferably a mass rate infusion, either mcg/kg/min or mg/kg/h. This helps smooth out some of the peaks and troughs in the level of sedation, as long as an initial bolus of propofol is given and an appropriate rate of infusion is administered. The pharmacokinetics of propofol is such that the infusion rate will need to be reduced over time, to maintain a stable plasma concentration, as redistribution to compartments V2 and V3 slows.
The main disadvantages of a manual infusion regime are as follows:
Bolus administration is not incorporated, either at the beginning or when rapid changes in depth of sedation are required
There may be no clear indication of the propofol dose being delivered if administered as a rate infusion (mls/h)
The estimated plasma propofol concentration being delivered is difficult to estimate, even for experts
Frequent changes in the drug infusion rate are required to maintain a stable plasma propofol concentration
It doesn’t guarantee a stable plasma and therefore effect-site propofol concentration
An increase in infusion rate without a bolus dose leads to a slow rise in plasma concentration
Published regimens are often not based on valid pharmacokinetic data
Clinical Example
A nearly 3-year-old boy, estimated weight 12-kg, has been brought into ED. The history is that he fell out of first floor window onto concrete. He cried immediately and an ambulance was called. His GCS is 15 and he needs a CT of his head, c-spine, thorax, and abdomen, but is too awake and for uncooperative for scanning without either sedation or general anesthesia. ABC are stable and do not require intervention and there are no other indications for endotracheal intubation. The decision is made to sedate with propofol for the scan . He has an IV in situ and is given midazolam 2 mg and a further 1 mg to settle him. A propofol bolus is titrated up to 20 mg total (1.67 mg/kg) and an infusion commenced a 5 mg/kg/h (83 mcg/kg/min). This is increased to 7.5 mg/kg/h (125 mcg/kg/min) after about 5 min and he sedates nicely. He is transferred to scan in a lightly sedated state and the scan is successfully completed after about 45 min.
The TivaTrainer© simulation (Fig. 26.2) demonstrates how the relatively small bolus of propofol leads to a rapid rise in the effect-site concentration to just under 2 mcg/ml concentration. The infusion leads to a very gradual rise in the plasma and effect-site concentrations, so that by the end of the scan they are both about 2 mcg/ml. As with opioids, there is a significant synergistic effect between propofol and midazolam, which allows a lower dose of propofol to be administered. It is also unnecessary to produce anything other than a moderate state of sedation for a quick CT scan, particularly as the patient needs to be awake rapidly at the end, to facilitate neurological status assessment.
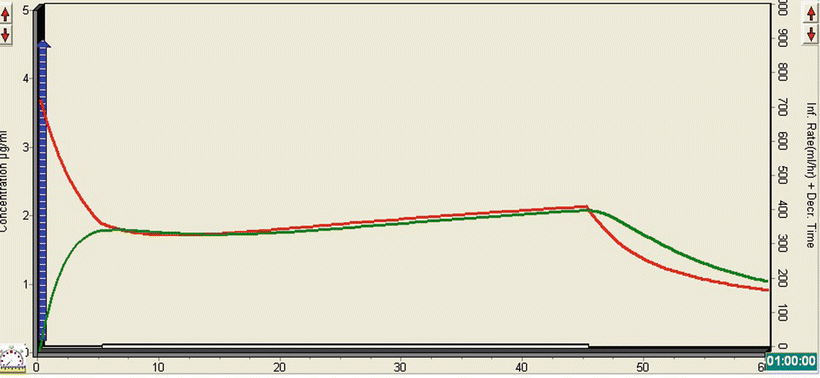

Fig. 26.2
TivaTrainer© simulation demonstrating the effect of a bolus dose of propofol followed by a continuous infusion on the plasma and effect-site concentration
In situations where a mass rate infusion is being used, rather than TCI, it is useful to be able to estimate the target propofol concentration being delivered to the patient. Table 26.9 shows the target propofol concentration likely to be achieved following bolus administration and at different infusion rates.
Table 26.9
TivaTrainer simulation in a 25 kg, 8-year-old boy, using the Paedfusor model with a fast ke0 value (0.8). The propofol bolus used was 3 mg/kg
Estimated propofol target concentration (μg/ml) | ||||||||
|---|---|---|---|---|---|---|---|---|
Propofol infusion rate (mg/kg/h) | Time (min) | |||||||
Bolus | 5 | 10 | 15 | 20 | 30 | 45 | 60 | |
2 | 4.3 | 3.2 | 1.8 | 1.3 | 1.1 | 1.0 | 1.0 | 0.9 |
4 | 4.3 | 3.4 | 2.1 | 1.6 | 1.5 | 1.5 | 1.5 | 1.5 |
6 | 4.4 | 3.6 | 2.4 | 2.0 | 1.9 | 1.9 | 2.1 | 2.2
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|





