 pp.100 and 101).
pp.100 and 101).
Hypoxia.
Hypovolaemia.
Hypo/hyperkalaemia (and other metabolic disorders).
Hypothermia.
Hypoglycaemia.
Tension pneumothorax.
Tamponade, cardiac.
Toxins.
Thrombosis (coronary or pulmonary).
Severe acidaemia.
Periods of physiological deterioration often precede within-hospital arrests.
1/3 of in-hospital cardiac arrests present with pulseless electrical activity (PEA), 1/3 with asystole, and 1/3 with ventricular fibrillation/pulseless ventricular tachycardia (VF/VT).
Features requiring immediate assessment include:
Sudden loss of consciousness
Impalpable pulse, or loss of arterial pressure trace
Obvious apnoea, or terminal gasping
Sudden loss of end-tidal CO2 trace
U&Es (to identify hypo/hyperkalaemia):
The fastest way of measure this is often via a blood gas analyser using an arterial or venous sample
Finger-prick blood glucose (hyperglycaemia).
ABGs (to identify profound acidaemia).
Echocardiography (tamponade, hypovolaemia, right ventricular hypertension in PE, regional wall motion abnormality, aortic dissection).
Equipment failure (ECG electrode failure or damped arterial trace).
Any cause of syncope or severe hypotension.
Adopt a SAFE approach (Shout for help, Approach with caution, Free from danger, Evaluate ABC); don gloves/PPE as appropriate.
Assess the patient: check for a response ‘shake and shout’:
If unresponsive call for help, position the patient on their back and open the airway (chin lift or jaw thrust)
Assess the breathing for up to 10 seconds (if ventilated confirm tube connections and tidal volume delivery)
At the same time manually confirm that the pulse is absent; do not rely on arterial pressure trace alone
If the patient has no signs of life, or is pulseless, or if there is any doubt, then commence CPR.
Ensure that the cardiac arrest team has been summoned (dial 2222).
ALS algorithms (Figs 4.1 and 4.2; pp.100 and 101) should be followed; do not delay defibrillation if appropriate.
pp.100 and 101) should be followed; do not delay defibrillation if appropriate.
Obtain information about the patient as soon as possible from notes, staff or relatives as this may help identify underlying cause and will clarify the resuscitation status of the patient.
Where doubt exists as about the patient’s resuscitation status CPR should be commenced
If a monitored cardiac VF/VT arrest is witnessed deliver 3 ‘stacked’ shocks, commencing CPR for 2 minutes immediately after the third.
Check the airway, and if in doubt about ETT or tracheostomy consider re-intubating or reverting to bag-and-mask ventilation.
If mechanically ventilated, turn the ventilator O2 to 100% and do not disconnect ventilator tubing during defibrillation:
Minimizing PEEP, if possible, may aid defibrillation
If CPR prevents the ventilator from working, switch to a self-inflating bag; this can also be left connected during defibrillation
Arterial pressure traces can be used to guide CPR; aim for a diastolic pressure >40 mmHg.
Drugs:
Whilst waiting for adrenaline bolus to be available consider increasing adrenaline/noradrenaline infusion rates
Other treatments:
If hypovolaemia is suspected IV fluids may be rapidly infused
Needle thoracocentesis/pericardiocentesis: for tension pneumothorax or cardiac tamponade ( pp. 546, 530, 146, and 80)
pp. 546, 530, 146, and 80)
Open-chest cardiac compression should be considered following cardiothoracic surgery or chest trauma
Echocardiography during the arrest may identify reversible causes.
ICU/HDU/CCU admission: it may be necessary to admit patients to a critical care setting for management of their underlying condition or post-arrest stabilization/treatments.
Any decision not to admit the patient following ROSC should be made by senior clinicians, where possible with the involvement of the patient’s relatives (and in light of the patient’s views, if known)
Transfer to a critical care environment is unlikely to be beneficial unless there is a period of cardiovascular stability
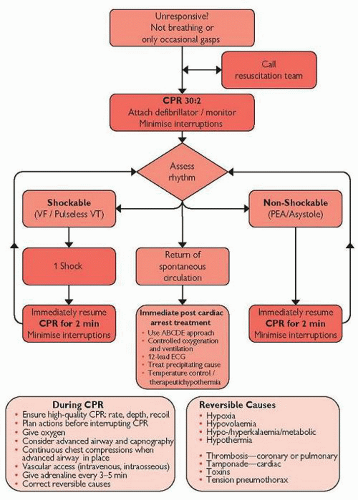
Fig. 4.2 Adult advanced life support algorithm. UK Resuscitation Council guidelines 2010. Reproduced with the kind permission from the Resuscitation Council (UK).
Surgical intervention: on occasion urgent surgery is required following ROSC (e.g. following trauma or ruptured ectopic pregnancy); surgical advice must be sought, and a decision taken as to whether to transport to theatre or operate within the current environment.
Angiography: where there is evidence of an acute ischaemic event it may be necessary to perform urgent angiography/coronary intervention; obtain an urgent cardiological opinion.
Thrombolysis: this may be necessary if a massive/submassive PE is evident (or after a coronary occlusion where angiography is not possible).
General support:
Continued mechanical ventilation: where this is considered appropriate aim for a SaO2 >94% and a PaCO2 of 4.5-5 kPa
Sedation: if required use short-acting drugs (e.g. propofol) so that neurological status can be rapidly assessed after discontinuation
Circulatory support: inotropic support or fluid therapy may be required depending on the underlying disease or degree of cardiac stunning; if LVF is present consider specific treatments ( p.122)
p.122)
Neurological support: employ measures to combat raised ICP (sedation, seizure, PaCO2 and glycaemic control; avoidance of hypoxia and hypotension; raising the bed-head to improve venous drainage)
Electrolytes: maintain blood glucose at 6-10 mmol/L; correct any hypomagnesaemia; maintain potassium at 3.5-4.5 mmol/L
Maintain haemoglobin of 8-10g/dL
Therapeutic hypothermia may be indicated (see Box 4.1)
Avoidance of pyrexia: if therapeutic hypothermia is not being employed, antipyretics, or surface cooling, may be necessary
Post cardiac arrest investigations should include: ABGs, FBC, U&Es, LFTs, blood glucose, cardiac enzymes, ECG, CXR.
Later investigations may also include echocardiography to assess cardiac damage and CT head to assess neurological damage
ALS is unlikely to be successful in cases where maximal supportive therapy is already being provided.
In patients who are hypothermic, or undergoing general anaesthesia, prolonged CPR may still be successful.
After CVC insertion, chest trauma or chest surgery do not forget to exclude tension pneumothorax or tamponade.
Hypo/hyperkalaemia and hypo/hypermagnesaemia are relatively common in critical care environments.
Pregnancy ( p.429): use a wedge or tilt to decrease aortocaval compression where possible, urgently arrange for obstetric help (Caesarean section may facilitate resuscitation and save the fetus).
p.429): use a wedge or tilt to decrease aortocaval compression where possible, urgently arrange for obstetric help (Caesarean section may facilitate resuscitation and save the fetus).
Hypothermia ( p.250): if first defibrillation is ineffective withhold further attempts until temperature is >30°C; actively re-warm.
p.250): if first defibrillation is ineffective withhold further attempts until temperature is >30°C; actively re-warm.
Drowning or trauma ( pp. 404 and 414): consider the possibility of cervical spine trauma, and drug or alcohol intoxication.
pp. 404 and 414): consider the possibility of cervical spine trauma, and drug or alcohol intoxication.
Prognostication of neurological recovery after a cardiac arrest is difficult; absence of pupillary and corneal reflexes at 72 hours (or 72 hours after cooling) is associated with a poor outcome.
Indications include comatose patients (GCS <9) with ROSC after a VF/VT out-of-hospital arrest with short ‘down-times’.
It may also be beneficial for other types of arrest in other settings.
Hypothermic patients.
Cardiovascularly unstable patients (including inotrope-dependent shock, septic shock, major haemorrhage).
Pregnancy.
Coagulopathy.
Rapid induction of cooling (e.g. an infusion of 20-30 ml/kg 0.9% saline or Hartmann’s).
Continued induction/maintenance using:
Peripheral cooling using exposure, tepid sponging and fans
Cooling blankets and mattresses
Cold IV fluid maintenance infusions
Use of intravascular cooling systems (i.e. CoolGardTM)
Trans-nasal evaporative cooling (e.g. RhinoChillTM)
Haemofiltration or cardiac bypass using cold fluids
Shivering may be controlled by deepening sedation and the use of neuromuscular blocking drugs (e.g. atracurium).
Monitor core body temperature, aiming for 32-34°C for 24-36 hours before rewarming at a rate of 0.25-0.5°C/hour.
Intravascular volume and electrolytes may require correction during cooling/rewarming.
Haemorrhage (e.g. trauma, aortic dissection, postoperative bleeding).
GI loss of fluids (e.g. vomiting, diarrhoea, high-output stoma).
Renal loss of fluids (e.g. diabetes insipidus, excessive diuretics).
Fluid redistribution (e.g. burns, trauma, major surgery, sepsis, posture).
Overdose of sedatives or vasodilators.
Spinal/epidural anaesthesia or analgesia.
Autonomic neuropathy (e.g. spinal cord lesion/trauma, Guillain-Barré).
Anaphylaxis.
Major vein compression (e.g. tumour, pregnancy, ascites, extensive intra-abdominal surgery).
Cardiac tamponade (e.g. pericardial bleeding, pericardial effusion).
Constrictive pericarditis.
↑intrathoracic pressure (e.g. tension pneumothorax, massive pleural effusion, mechanical ventilation).
PE.
Hepatic failure.
Thyrotoxicosis.
Myxoedema coma.
Adrenal insufficiency.
Poisoning (e.g. cyanide, carbon monoxide).
Anxiety and sweating.
Tachypnoea, dyspnoea; Kussmaul’s breathing (if hypoperfusion causes reduced tissue perfusion and a metabolic acidosis).
Hypoxia, cyanosis.
Tachycardia (bradycardia may be a pre-terminal sign, or be associated with a arrhythmia).
Cardiac hypoperfusion: angina, or ECG evidence of ischaemia.
Renal hypoperfusion: oliguria/anuria, raised urea and creatinine.
Neurological hypoperfusion: confusion, syncope, ↓GCS.
Cold peripheries, poor peripheral perfusion and delayed capillary refill.
Thready pulse with reduced pulse pressure.
↓cardiac output (cardiac index <2.2 L/minute/m2 with adequate preload).
↑systemic vascular resistance.
In hypovolaemic shock JVP/CVP is ↓; there may be evidence of fluid or blood loss, or venous pooling.
Examination of the lungs may show evidence of pulmonary oedema
Bounding pulse with wide pulse pressure.
Strong apical cardiac impulse.
Normal or ↑ cardiac output.
↓systemic vascular resistance.
Depending on the degree of skin perfusion peripheries may or may not be warm; capillary refill may be brisk or reduced.
Pyrexia and raised WCC where there is associated sepsis.
ABGs (metabolic acidosis, hypoxia, compensatory hypocapnia).
Coagulation screen (deranged with haemorrhage or DIC).
Serum glucose (hypo/hyperglycaemia).
LFTs (deranged with ischaemia or liver failure).
Cardiac enzymes (if cardiac ischaemia suspected).
Blood, urine, sputum culture (if infection suspected).
12-lead ECG (cardiac ischaemia, arrhythmias).
CXR (evidence of infection, cardiomegaly, pulmonary oedema).
Echocardiography (cardiac tamponade, hypovolaemia, RV hypertension in PE, regional wall motion abnormality, aortic dissection).
Cardiac output monitoring (pulmonary artery catheterization, TOD, or pulse contour analysis may assist in differentiating between low cardiac output or low SVR states).
Damped arterial pressure traces or poorly fitting BP cuffs can giving spurious readings, if in doubt re-check manually.
Mild ‘hypotension’ may be a normal finding in fit healthy individuals.
Give 100% O2.
The airway may be compromised due to impaired conscious level:
Intubate the trachea as appropriate
If this is impossible (i.e. in a remote location) the patient can be placed in the recovery position (depending on other injuries)
Ensure breathing/ventilation is adequate:
In severe shock ventilatory support (non-invasive or invasive) may be needed to optimize oxygenation, especially in fatigued patients
If pulmonary oedema is present treat appropriately ( p.86)
p.86)
Assess hypotension: questions which need to be answered include:
Treat any identifiable underlying cause requiring immediate attention:
Tension pneumothorax ( p.80)
p.80)
Cardiac tamponade ( p.146)
p.146)
Arrhythmias ( pp.132 and 138)
pp.132 and 138)
Major haemorrhage ( p.112)
p.112)
Anaphylaxis ( p.110)
p.110)
Take a focused clinical history, carefully examine the patient, and review notes/charts where possible; identify the likely cause.
 p.112):
p.112):
Fluid resuscitate and treat underlying cause or proceed to surgery.
Ensure IV access (typically 2 large-bore cannulae).
Initial resuscitation is usually in the form of crystalloids/colloids or warmed blood in the case of haemorrhage, given rapidly until there are signs of adequate filling (restoration of MAP, neurological function, urine output; normalization of tachycardia).
In haemorrhage awaiting source control (e.g. ruptured AAA, penetrating trauma) limited resuscitation targets may be necessary: palpable radial pulse, systolic BP ≤80 mmHg (‘permissive hypotension’).
Inotropes/vasopressors can be used but may mask hypovolaemia.
 p.108): treat underlying cause and commence fluid resuscitation, with/without vasopressor therapy:
p.108): treat underlying cause and commence fluid resuscitation, with/without vasopressor therapy: p.122):
p.122):
Record 12-lead ECG and compare to previous ECGs, assessing for evidence of myocardial infarction/ischaemia ( p.126).
p.126).
Aggressive fluid therapy is not recommended in cardiogenic shock, although up to 500 ml can be given initially.
Admit patients with refractory hypotension (unresponsive to moderate fluid resuscitation) into a suitable critical care facility.
Continue respiratory support until cardiovascular stability is achieved.
Arterial and/or central venous cannulation are likely to be needed.
Monitoring may be needed to obtain information about:
Ventricular filling (e.g. CVP, echocardiography, pulmonary artery catheterization)
Cardiac output and SVR (e.g. echocardiography, oesophageal Doppler, pulse contour analysis, pulmonary artery catheterization)
Optimize preload using crystalloids, colloids (or blood if required).
Consider optimizing cardiac contractility using inotropes (e.g. adrenaline, dobutamine).
Optimize SVR using vasoconstrictors (noradrenaline) or vasodilators (glyceryl trinitrate).
Correct any electrolyte imbalance and severe metabolic acidosis (renal replacement therapy may become necessary).
If there is no response to volume replacement, consider the possibility of spinal shock ( p.409) or adrenal insufficiency (
p.409) or adrenal insufficiency ( p.236).
p.236).
See Table 4.1 for drugs used to treat hypotension.
There is no ‘normal’ or ‘average’ CVP number, the measurement is determined by venous return and right ventricular compliance.
CVP measurement should be regarded as a trend.
It is conventional to volume load an under-resuscitated patient to a target CVP of approximately:
8-10 mmHg in non-ventilated patients
12-16 mmHg in ventilated patients
But CVP monitoring is dynamic, i.e. patients should be volume-loaded until a rise in CVP (or cardiac output) is sustained:
Repeated fluid challenges are likely to be required in shocked patients with low intravascular volume with compensatory vasoconstriction (initial rises in CVP or cardiac output are often transient)
Vasodilatory challenges (e.g. general anaesthesia) will cause the CVP to fall
Low values (<65%) are associated with a worse outcome in trauma, severe sepsis, myocardial infarction, and cardiac failure.
ScvO2 may be ‘artificially’ high in the presence of a significant shunt.
It is conventional to resuscitate patients to a target ScvO2 of >70%.
Monitoring should be dynamic to ensure that any rise is sustained.
Hypothermia.
Vasoconstriction, or vasoconstrictive drugs (e.g. noradrenaline).
Sympathetic stimulation.
Sepsis.
Hyperthermia.
Hyperthyroidism.
A-V shunts.
Liver failure.
Vasodilators.
Spinal/epidural block or spinal shock.
A MAP of 65 mmHg in a young fit patient (e.g. due to an epidural) may cause no tissue hypoperfusion, whereas in patients with vascular disease it may lead to myocardial ischaemia (coronary blood occurs mainly in diastole), cerebral ischaemia, or acute pre-renal failure.
Overlapping aetiologies and mixed pictures occur (e.g. sepsis with myocardial failure).
Table 4.1 Drugs used to treat hypotension | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Insect bites (especially wasp and bee stings).
Foods and food additives (especially peanuts, fish, eggs).
Drugs and IV infusions, especially:
Antibiotics and vaccines
Colloids and blood products
IV contrast media
Pabrinex, parenteral vitamin K
Anaesthetic induction agents, neuromuscular blocking drugs
Latex allergy.
Idiopathic (no readily identifiable cause).
Cardiovascular collapse (75% of cases): cardiac arrest; or tachycardia, hypotension, and other signs of shock ( p.104).
p.104).
Bronchospasm (40%): wheeze, cough, and/or accompanying desaturation, tachypnoea and dyspnoea.
Angioedema (12%): leading to laryngeal oedema and/or airway obstruction/stridor.
Cutaneous signs (72%): erythema, cutaneous rash, urticaria.
Take blood samples for mast cell tryptase level:
As soon as possible after treatment of initial reaction, and
1 hour after the reaction, and 6-24 hours after the reaction
ABGs (metabolic and/or respiratory acidosis).
FBC.
Clotting studies, fibrinogen (deranged with associated DIC).
CXR (to exclude differentials).
Airway/ETT obstruction, or endobronchial intubation.
Tension pneumothorax/haemothorax.
Air embolus/amniotic fluid embolus/fat embolus.
Severe bronchospasm/asthma.
Distributive shock (e.g. sepsis/neuraxial blockade/spinal cord injury).
Localized cutaneous reactions (e.g. type IV allergy: T cell mediated 6-48 hours after allergen exposure).
Hereditary angioneurotic oedema.
Thyroid crisis or carcinoid syndrome.
Stop trigger agents and call for help.
Give 100% O2.
Secure the airway and ensure breathing/ventilation is adequate; early endotracheal intubation may be required, especially in angio-oedema.
If already intubated exclude airway/breathing system obstruction.
Lie patient flat with legs elevated.
IM dose 0.5-1 mg (0.5-1 ml of 1:1000) up to every 10 minutes
IV dose 50-100 mcg (0.5-1 ml of 1:10 000) over 1 minute, and repeat as necessary (only give if you are familiar with IV adrenaline usage)
Continuous ECG monitoring is advisable whilst giving adrenaline
Ensure IV access and commence rapid infusion of crystalloid.
Adrenaline infusions may be required.
If hypotension persists/relapses (5% of cases) start inotrope infusions.
Deflate the ETT cuff prior to extubation in order to ascertain a leak (to gauge the degree of airway oedema).
Refer the patient to an immunologist; include copies of the notes, drug chart, and a full description of the reaction chronology.
Serum IgE and skin-prick tests will be required.
Report anaphylactic reactions on a CSM ‘yellow card’.
If this was a ‘wrong blood’ reaction, send all products back to the lab and involve a haematologist.
Monitor for a 2° deterioration or biphasic response.
Myocardial infarction and arrhythmias can occur, especially if there is pre-existing ischaemic heart disease, or if hypoxia/acidosis is present.
Stridor may be mistaken for bronchospasm, and vice versa.
Do not forget that colloids and latex can cause anaphylaxis:
Latex anaphylaxis may present up to 30-60 minutes after an event due to delayed airborne exposure or mucous membrane contact.
Trauma.
GI bleeding.
Surgery or postoperative/post-procedure bleeding.
Bleeding in response to trivial trauma, associated with:
Coagulation factor abnormality (e.g. haemophilia)
Thrombocytopaenia
Splenomegaly
Other occult bleeding (thoracic, intra-abdominal or retroperitoneal; e.g. ruptured aortic aneurysm or ruptured ectopic pregnancy).
There may be a bleeding site, or blood in postoperative drains.
Certain mechanisms of trauma can cause occult bleeding ( p.411).
p.411).
Certain patients are more likely to have a coagulopathy ( p.302):
p.302):
Patients receiving anticoagulant therapy
Patients with a history of congenital disease (may carry a card)
Patients being treated for haematological malignancy
Slight tachycardia and mild peripheral vasoconstriction (mildly ↑diastolic BP and narrowed pulse pressure).
Thirst may occur as accompanying symptom.
Vasoconstriction with clearly raised diastolic BP, narrowed pulse pressure and delayed capillary refill (>2 seconds).
Oliguria.
Patient may be anxious.
Systolic hypotension develops; pulse becomes thready.
Dyspnoea develops.
Agitation ensues.
Severe hypotension.
Anuria.
Ashen complexion.
Drowsiness or unconsciousness.
Diagnostic (to identify the bleeding source):
Plain radiographs (especially of pelvis and chest following trauma)
Abdominal US (may identify free blood, ruptured ectopic pregnancy, or aortic aneurysm)
CT scan, of pelvis, abdomen, or chest according to clinical indication
Diagnostic peritoneal lavage
Endoscopy (for suspected GI bleeding)
Angiography (may be diagnostic and/or potentially therapeutic)
Radio-labelled bleeding scan (may be considered if source unclear)
β-HCG, urine or blood (may aid in the diagnosis of ectopic pregnancy)
To monitor progress and identify the development of complications:
Other causes of shock associated with trauma (e.g. tension pneumothorax, cardiac tamponade, cardiac contusion, spinal cord lesion).
Hypovolaemic shock 2° to fluid losses other than blood:
Profound dehydration (e.g. DKA, vomiting, diarrhoea, diuresis)
Massive ‘third-space’ losses (e.g. profound ileus, sepsis, major burn)
Cardiogenic shock; left or right ventricular failure (e.g. myocardial infarction, aortic stenosis, hypertrophic cardiomyopathy, PE).
Distributive shock (e.g. sepsis, anaphylaxis, neuraxial blockade).
In cases of trauma ensure cervical spine protection.
Secure the airway and ensure breathing/ventilation is adequate; endotracheal intubation may be required.
Assess the degree of blood loss and control haemorrhage if possible:
Compression/elevation of external bleeding sites, or use of a tourniquet in life-threatening haemorrhage from a limb
The patient may require immediate life-saving surgery (e.g. ectopic pregnancy, ruptured spleen)
Stabilize fractures
Consider endoscopic or interventional radiological procedures
Reverse the effects of any anticoagulants if they are present (e.g. using vitamin K IV, or prothrombin complex IV)
Commence circulatory support in shocked patients:
Head-down tilt (Trendelenburg) may be required
In difficult cases the use of femoral veins, external jugular veins may be required; or an IV cutdown at the ankle or antecubital fossa ( p. 540) or insert IO access
p. 540) or insert IO access
Take a sample for blood crossmatching when inserting cannulae
Start restoring intravascular volume ( p.116)
p.116)
Commence blood transfusion1 alongside crystalloids/colloids as soon as possible if bleeding is >30%TBV, >1.5 L, or Hb <8 g/dl with ongoing haemorrhage
O-negative or type-specific blood may be required in severe haemorrhage whilst waiting for crossmatched blood
In haemorrhage awaiting source control (e.g. ruptured AAA, penetrating trauma) limited resuscitation targets may be necessary: palpable radial pulse, systolic BP ≤80 mmHg (‘permissive hypotension’).
Use inotropes/vasopressors with caution (they may mask under-resuscitation).
Where there is massive blood loss (≥6 units of blood or ≥30% TBV lost) involve haematologists for guidance on appropriate blood and component therapy; common protocols include the following:
FFP (12 ml/kg; or approximately 2 bags) if PT or APTT >1.5 × normal, or if more than 4-6 units of stored blood is transfused
Platelets (0.5-1 units/10 kg body weight; or 1-2 adult doses) if the count is <50 × 109/L
Cryoprecipitate (1 pack/10 kg body weight) if fibrinogen <0.8 g/L
Repeat coagulation studies every 2 hours, or after every 4-6 units of packed red cells
Consider using recombinant factor VIIa.
Cell-salvage may be available in some settings.
Many centres now have ‘major haemorrhage packs’ consisting of various quantities of packed red cells, fresh frozen plasma, cryoprecipitate, and platelets for use in the event of massive transfusion requirements.
Look for and treat complications of massive blood transfusion (e.g. hypothermia, hypocalcaemia, hyperkalaemia, coagulation factor depletion, thrombocytopaenia, metabolic acidosis).
Some or all of the following monitoring is likely to be needed:
CVC to assess intravascular fluid status
Urinary catheterization to allow urine output measurements
Arterial line insertion for invasive BP measurements
CO monitoring to assess cardiac contractility and vascular resistance
Inotropic support may be required, but this should be discontinued as soon as volume replacement and the control of bleeding allows; adrenaline or noradrenaline are the initial agents of choice.
The contact name and telephone number for doctor in charge
Location of patient and any known patient details:
Name, Date-of-Birth, Gender
Identification no., Estimated weight, ABO/Rh group
Type and volume of blood components required
Send pre-transfusion screening sample for group and antibody screen, and FBC and coagulation studies.
Send request forms for any blood products (including O-negative)
Red cells needed immediately?
Use emergency O-negative from designated fridge or blood bank
Red cells needed in 15 minutes?
ABO/RhD group-specific available within 15 minutes of receiving sample; retrospective full crossmatch carried within 30 minutes
Red cells needed in 45 minutes?
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



 p.94
p.94 p.395
p.395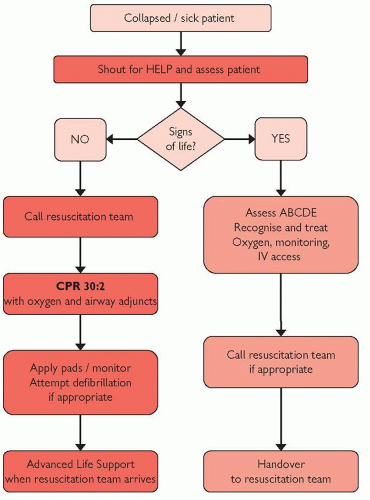
 p.122
p.122