Fig. 26.1.
Two-hit theory on cause of chronic groin and scrotal content pain (From Brahmbhatt et al. [3], with kind permission Springer Science + Business Media).
CGSCP may affect over 100,000 men annually [4, 5]. Prevalence can range up to 33 % of men after vasectomy [6] and 63 % after inguinal hernia repair [7–9]. After hernia repair, the pain can be neuropathic or non-neuropathic secondary to mesh. Even with such a high prevalence after hernia repair, only 1 % of patients who suffer from CGSCP may be referred for further evaluation [10]. In this chapter we will review the current literature and present a structured algorithm for the evaluation and management of CGSCP.
Anatomy and Function
Embryology
At 7–8 weeks gestation, gonads differentiate into testis in the posterior abdominal cavity. After 8 weeks, through the influence of hormones, the testicles begin their descent into the scrotum. Until 7 months, they remain near the inguinal canal. Before birth or within a few weeks after birth, the testicles complete their descent into the scrotum. As the testicle descends from the abdominal cavity, it brings with it layers of the peritoneum and abdominal wall. Figure 26.2 lists the layers from external to internal order. It is important to note that the external spermatic fascia is derived from external oblique fascia, cremasteric muscle from internal oblique fascia, internal spermatic fascia from transversalis fascia, and tunica vaginalis from peritoneum. If the descent does not occur completely, then patients are labeled as having undescended testicles, which may require medical or surgical intervention.
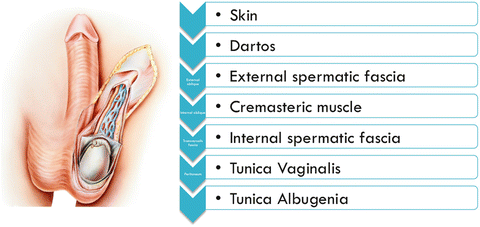

Fig. 26.2.
Layers of the scrotal wall.
Anatomy
The testicle is egg shaped with an average length of 5 cm. Testicular function is tightly regulated with signaling from the hypothalamus and anterior pituitary gland. The main function of the testicle is to produce sperm from germ cells and testosterone from Leydig cells . There is another cell type (called Sertoli cells ) that is important for support. Sperm travels through the testicle (lobules, seminiferous tubules, rete testis, epididymis) and vas deferens until it mixes with fluid from the seminal vesicles and prostate to form semen. This combined fluid is eventually expelled into the urethra during ejaculation.
The spermatic cord houses the testicular artery, testicular veins (pampiniform plexus), vas deferens, artery of vas deferens, lymphatic vessels, and nerves. Neural innervation to the testicle is via a complex neural network with significant crossover. Afferent innervation of the scrotum originates via somatic nerves in the genital branch of the genitofemoral nerve, ilioinguinal nerves, and autonomic branches from T10-L1 parasympathetic ganglia [11]. The genitofemoral and ilioinguinal nerves provide anterior scrotal wall and thigh innervation. The posterior scrotal wall is innervated via the perineal branch of the pudendal nerve. There is an alternate autonomic pathway between the pelvic plexus and testis via the vas deferens, which explains the positive response to anesthetic injections to the pelvic ganglia [12]. On average, there are 31 small diameter (less than 1 mm) nerve fibers in the spermatic cord. The three primary sites (trifecta nerve complex) of highest nerve density are (in decreasing order): cremasteric muscle fibers, perivasal tissue and vasal sheath, and posterior peri-arterial/lipomatous tissue [13].
Evaluation
Workup of CGSCP begins with a thorough history and physical examination. The characteristics of pain, including onset, duration, and severity, are questioned. Pain is rated using the visual analog scale and externally validated pain impact questionnaire (PIQ-6, Quality-Metrics Inc., Lincoln, RI, USA).
Physical examination focuses on the groin and testicle in the attempt to identify any anatomic causes of the pain, including hernia, varicocele, testicular masses, epididymal cysts, and granulomas from previous vasectomy. All possible causes such as ureteral stones, infection (orchitis or epididymitis), or back problems (lumbar disk hernia) need to be ruled out. Urine analysis, scrotal ultrasonography, abdominal computerized tomography (CT), and spinal magnetic resonance imaging (MRI) should be performed when indicated. Scrotal ultrasound is not necessary when physical examination and urine analyses are normal in patients with chronic scrotal pain. Van Haarst et al. evaluated scrotal ultrasonography imaging of 111 chronic scrotal pain patients with normal physical examination and urine analyses and found 12 epididymal cysts less than 0.5 cm and three subclinical varicocele but no clinical significant abnormalities [14]. Since a significant percentage of CGSCP is idiopathic, patients often have completely negative evaluations. Treatment for these patients is initiated using a structured algorithm (Fig. 26.3).
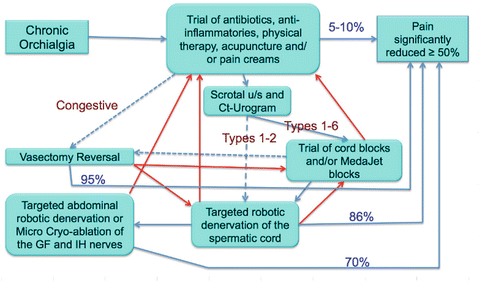

Fig. 26.3.
Algorithm for evaluation and management of chronic groin and scrotal content pain.
Medical Treatment
In the absence of any acute findings that require surgical intervention, conservative medical therapy is a first-line treatment [15]. One month of nonsteroidal anti-inflammatory drugs (NSAIDs) is recommended [1]. We usually start with meloxicam 7.5 mg daily or high-dose ibuprofen 600 mg orally three times daily. Newer low-dose NSAIDs such as Zorvolex 35 mg BID-TID can be used to decrease side effect potential.
Sexually transmitted infection with gonorrhea or chlamydia should be considered in men between the ages of 15–35. This is usually treated with azithromycin 1 g orally once (or doxycycline 100 mg orally twice daily for 10 days) and ceftriaxone 125 mg intramuscularly once. Over the age of 35, Escherichia coli is a common urinary pathogen that can cause epididymal infection. E. coli infection can be treated with a course of quinolones (ciprofloxacin 500 mg orally twice daily or levofloxacin 500 mg orally once daily for 10 days). A 1-month supply of quinolone therapy can also be considered in combination with NSAIDs for refractory cases.
Antidepressants used at lower doses for chronic pain work by inhibiting the reuptake of norepinephrine. A commonly used class of antidepressants is tricyclics, which include amitriptyline 10–25 mg orally daily and nortriptyline 10–150 mg orally daily. These medications are slowly titrated up to therapeutic levels. The use of these medications can also help the comorbid psychological factors that may contribute to genital pain. In a study of 48 patients with genital pain and no organic findings, psychological disorders were diagnosed commonly, including major depression (27 %), somatization disorder (56 %), and chemical dependency (27 %) [16]. It is critical to taper patients off tricyclic antidepressants—they should never be stopped abruptly.
Anticonvulsants contribute to pain management through their ability to modulate central calcium channels with chronic use via an effect on trafficking [17]. Gabapentin and pregabalin are common anticonvulsants used for neuropathic pain. We start with gabapentin 300 mg orally three times daily and may titrate up as needed. Patients tend to respond well to these medications, but its frequent dosing and side effect profile lead to a high dropout rate. If patients are placed on chronic medications, a multidisciplinary approach to their follow-up is generally recommended.
Spermatic Cord Block
Spermatic cord nerve blocks using local anesthetic with or without steroids can be therapeutic and diagnostic. In our practice, we mix 15 mL 1 % lidocaine, 15 mL 0.25 % marcaine, and dexamethasone 4 mg. The blocks target three areas of high nerve density [13]. First, high-pressure injection of the perivasal tissue is done using a Medi-Jet. Another 5 mL, using a needle, is directly injected directly around the perivasal tissue. We then inject 10 mL medially to the external inguinal ring to target the branches of the ilioinguinal nerve and 10 mL laterally to the external inguinal ring to target genital branches of the genitofemoral nerve.
In patients with no apparent etiology, the blocks can provide temporary relief while helping to predict a positive response to surgical interventions. Benson et al. demonstrated that a positive response to spermatic cord block helps predict a durable and complete resolution of symptoms after microsurgical denervation of the spermatic cord (MDSC) [18]. They achieved an average 89 % decrease in pain with spermatic cord block for median 8 h (1–168 h) in 74 men (77 testicular units). Also improvement from the spermatic cord block was a predictor of overall improvement after MDSC (p = 0.05).
Microsurgical Targeted Denervation of the Spermatic Cord
Introduction
Microsurgical targeted denervation of the spermatic cord (MDSC) is a minimally invasive surgical option for the management of CGSCP after conservative treatments have failed [2, 19]. An animal study to evaluate the effectiveness of the MDSC procedure showed a significant decrease in median number of nerve fibers remaining around the vas deferens after MDSC procedure, compared to sham (MDSC = 3.5 nerves, sham = 15.5 nerves, p = 0.003) [20]. In this procedure (described in detail below), we target tissue in or around the spermatic cord that carries high-density nerve fibers that contribute to chronic pain. Within these nerve fiber s, there are significant anatomic and pathologic differences (Wallerian degeneration) compared to controls (Fig. 26.4) [3, 13]. Targeting these specific areas leads to the preservation of a significant portion of the spermatic cord and potentially fewer complications.
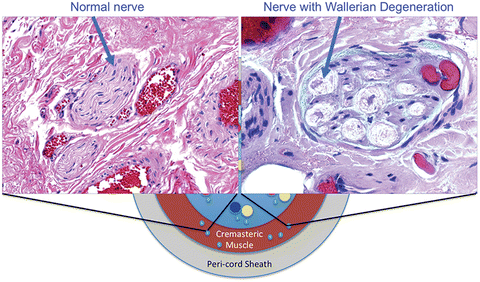

Fig. 26.4.
Nerve fiber with and without Wallerian degeneration on H&E staining (From Brahmbhatt et al. [3], with kind permission Springer Science + Business Media).
We published our most updated results of the 546 robotic targeted MDSC (RTMDSC) procedures for chronic groin pain [21]. On the last review of our data our total has increased to over 620 patients. Mean preoperative duration of orchialgia in our patients is 2.4 years.The median robotic operative duration was 20 min (range, 10–150 min). Using the externally validated pain assessment tool PIQ-6, we assessed preoperative and postoperative pain. At 6 months there was a 71 % significant reduction in pain and 72 % significant reduction at 1 year. Using the visual analog pain scale, there was an 85 % significant reduction in pain (63 % complete resolution and 22 % greater than 50 %). Complications were limited to one testicular ischemia, two testicular artery injuries (repaired intra-op with no long-term sequelae), one vasal injury (repaired intra-op with no long-term sequelae), 11 hematomas, three seromas, and five wound infections.
There are several advantages to using the robotic platform, which includes improved visualization, decreased tremor, and less dependence on a surgical assistant. Robotic-assisted MDSC seems safe and feasible, and the outcomes appear promising for durable relief.
Technique in Detail
A 1–2 cm transverse subinguinal incision is made. The incision is carried down until the spermatic cord is reached. The spermatic cord is brought up to the surface. Posterior medial and lateral dissection and cauterization are performed to ligate branches of the ilioinguinal and genitofemoral nerves in this area.
The robot is positioned over the patient. A 0° camera lens is utilized. The right, left, and the fourth robot arms are loaded with Black Diamond microforceps, Maryland bipolar grasper, and monopolar curved scissors, respectively (Fig. 26.5) [3]. If a flexible CO2 laser fiber is used for dissection, then the fourth arm is replaced with a Black Diamond microforceps to hold the flexguide laser holder (Fig. 26.6) [3].
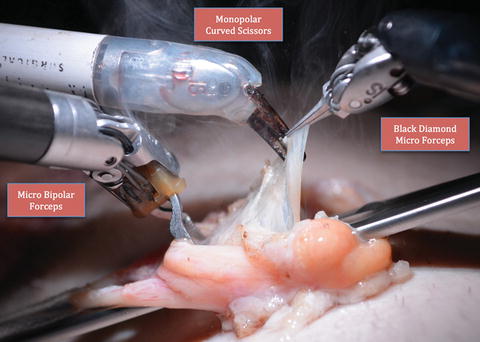
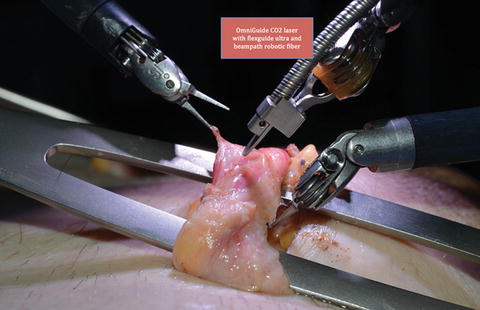

Fig. 26.5.
Standard robotic instrumentation for targeted denervation (From Brahmbhatt et al. [3], with kind permission Springer Science + Business Media).

Fig. 26.6.
Flexible CO2 laser instrumentation during targeted denervation (From Brahmbhatt et al. [3], with kind permission Springer Science + Business Media).
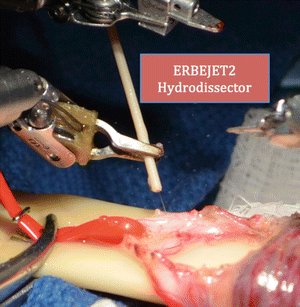
The anterior cremasteric muscle is divided. The presence of a testicular artery is confirmed with real-time intraoperative micro-Doppler (Vascular Technology Inc, Nashua, NH). The posterior cremasteric fibers and posterior fat component are ablated. The vas is isolated, and generally the artery and vein to the vas are dissected away from the vas. The perivasal tissue is now ablated. Hydrodissection of the perivasal tissue is now performed (Fig. 26.7) [3], using the ERBEJET 2 hydrodissector (ERBE Inc., Atlanta, GA) to ablate residual nerve fibers.