AMYOTROPHIC LATERAL SCLEROSIS
Amyotrophic lateral sclerosis (ALS), often called Lou Gehrig’s disease, causes rapidly progressive muscle atrophy and weakness resulting from the degeneration of both upper and lower motor neurons. ALS leads to varying degrees of spasticity, hyperreflexia, and muscle paralysis, eventually resulting in pulmonary complications and the need for mechanical ventilatory support. Because there is no cure, clinicians attempt to slow disease progression and preserve function as much as possible. Medical management is directed at preventing pulmonary infections and forestalling terminal respiratory failure.
Since 2009, 13 genes and loci have been identified that are associated with the disease.1 Inclusions in the TAR DNA-binding protein-43 have been found in both ALS and frontotemporal dementia.2 Environmental exposures are suspected to increase the risk of ALS, but no specific ones have been identified to date.3
Gross CNS pathology includes frontal cortical atrophy, degeneration of both the corticospinal and spinocerebellar tracts, a reduction in large cervical and lumbar motor neurons, and cranial nerve nuclei degeneration. Both motor and sensory peripheral nerves undergo axonal degeneration and segmental demyelination, including motor end plate and axon terminal involvement.
Upper motor neuron demyelination and dysfunction cause limb spasticity, hyperreflexia (including Babinski sign and a brisk jaw-jerk reflex), and emotional lability. Limb weakness, a lower motor neuron dysfunction, is the first symptom in 65% of patients.4 Other associated lower motor neuron dysfunctions include atrophy, cramps, fasciculations, dysarthria, dysphagia, and difficulty in mastication. At the time of initial presentation, asymmetric extremity cramping, fatigue, weakness, muscle fasciculations, and atrophy can be seen, especially in the upper extremities.5 Facial weakness, dysarthria, tongue weakness, atrophy, and fasciculations can be seen with bulbar lower motor neuron dysfunction. Despite these profound motor findings, sensory and cognitive function is usually spared. Regardless of the initial symptoms, widespread motor and respiratory dysfunction progresses within weeks to months. Significant extremity atrophy occurs, as well as fasciculations, hyperreflexia, foot drop, and claw deformity of the hand. Patients also may develop monotonous speech caused by tongue atrophy, despite the relative sparing of facial and eye movements. Some patients eventually diagnosed as having ALS present initially with cervical or back pain consistent with an acute compressive radiculopathy. Despite successful operative intervention, significant muscle wasting consistent with motor neuron dysfunction develops shortly after the procedure.6 Progressive respiratory muscle weakness initially causes exertional dyspnea and eventual dyspnea at rest. Overt dementia and parkinsonism may occur in up to 15% of patients, especially those with ALS. Other cognitive problems, such as apathy, poor attention and motivation, and altered social skills, may be noted.
The clinical diagnosis of ALS is suggested when there are signs of both upper and lower motor neuron dysfunction, including weakness, muscle atrophy, fasciculations, and hyporeflexia without other CNS dysfunction.7 Given the complexity of diagnosing ALS, the median time to diagnosis is 14 months.1 ALS-like symptoms can be seen with other systemic illnesses, such as diabetes, dysproteinemia, thyroid and parathyroid dysfunction, vitamin B12 deficiency, heavy metal toxicity, and vasculitis, as well as CNS and spinal cord tumors. The diagnosis requires the exclusion of other inflammatory neuropathies, such as myasthenia gravis. Refer patients with signs suggesting ALS to a neurologist for definitive diagnosis. The Amyotrophic Lateral Sclerosis Functional Rating Scale (revised) is reliable for diagnosis of ALS.8 Electromyography, nerve conduction velocity studies, spinal fluid analysis, and neuromuscular biopsies are useful diagnostic studies. MRI excludes other disease processes but does not confirm the diagnosis of ALS.9
Therapy is designed to enhance muscle function, especially those that support breathing, swallowing, and speech, in order to avoid malnutrition, recurrent aspiration, or choking. Riluzole, which modulates the excitotoxin glutamate, may prolong survival.10,11,12,13 It is most useful in patients with a clear diagnosis of ALS whose symptoms have been present for <5 years, with a forced vital capacity >60% of predicted, who do not have a tracheostomy. Guidelines on care and treatment of ALS patients are available.12,13
Optimizing pulmonary function, including the eventual use of long-term assisted ventilation, is an important part of enhancing the quality of life as diaphragm weakness progresses.12 Many ALS patients will find some benefit from regular progressive resistance exercise, which may provide an anti-inflammatory effect, a neuroendocrine effect, or beneficial effects on CNS plasticity and myofiber remodeling.14
Typically, patients with ALS will not present to the ED undiagnosed unless there is extremely rapid disease progression or a long period without medical care. Emergency management usually is required for acute respiratory failure, aspiration pneumonia, choking episodes, or trauma related to extremity weakness. Blood gas determination does not reliably predict impending respiratory failure, because mild hypoxia and hypercarbia may exist throughout the disease course. A forced vital capacity <25 mL/kg, or a 50% decrease from predicted normal, increases the risk of aspiration pneumonia and respiratory failure. Provide treatment to improve pulmonary function (e.g., nebulized medications, steroids, antibiotics, assisted ventilation, and intubation). Because the need for long-term ventilatory assistance rarely reverses, establish or confirm the patient’s preference regarding intubation through patient and family conversation, a living will, or power of attorney for health care. Hospital admission is indicated with impending respiratory failure, pneumonia, the inability to control secretions, or a worsening overall status that requires social service intervention for long-term placement.
MYASTHENIA GRAVIS
Myasthenia gravis is an autoimmune disease characterized by muscle weakness and fatigue, which is seen especially with repetitive use of voluntary muscles. Acetylcholine receptor antibodies impair receptor function at the neuromuscular junction, causing muscle weakness, most often in proximal muscles. This weakness is generally relieved by rest and requires long-term immunotherapy. The diagnosis of cholinergic and myasthenic crises and the aggressive management of respiratory complications associated with a myasthenic crisis are the most important issues for the ED management of myasthenia gravis patients. Occasionally a first diagnosis of myasthenia gravis can be suspected in the ED if increasing muscle weakness is noted during the ED stay or is reported by the patient to occur over the day.
In the normal neuromuscular junction, acetylcholine release by the nerve fiber causes a localized end plate potential that leads to muscle fiber contraction. In myasthenia gravis, there is a marked decrease in the number and function of the muscle fiber acetylcholine receptors, despite normal nerve anatomy and function. Failure to respond to acetylcholine stimulation causes decreased muscle fiber potential amplitudes, leading to decreased muscle strength. Acetylcholine receptor autoantibodies are seen in approximately 80% of patients.15 These antibodies react with the acetylcholine receptor. Disease severity can be correlated with acetylcholine receptor autoantibody levels. The autoantibodies cause accelerated acetylcholine receptor degradation, dysfunction, and blockade.
Either dysfunction of the thymus gland or an immune response to exogenous infectious antigens causes the pathologic autoimmune response. The thymus is abnormal in most patients with myasthenia gravis, most often as thymic hyperplasia or a thymoma. Thymectomy resolves or improves the symptoms in most patients, especially those with a thymoma. It is likely that the acetylcholine receptor autoantibodies arise after exposure to similar antigens, such as those caused by herpes simplex virus or bacterial infection, causing a pathologic attack on the acetylcholine receptor proteins.
The symptoms of myasthenia gravis can mimic the symptoms seen in many other chronic neurologic disorders, and some call it “the great imitator.” Most myasthenia gravis patients have general weakness, especially of the proximal extremity muscle groups, neck extensors, and facial or bulbar muscles. Although ptosis and diplopia are the most common presenting symptoms, limb weakness and oropharyngeal symptoms, such as dysphagia, dysarthria, dysphonia, and dyspnea, can also be seen initially or occur over time. Ocular signs can include Cogan lid twitch (in which the eyes are lowered for 10 to 20 seconds, then droop or twitch when the patient attempts to raise them); ptosis; cranial nerve III, IV, or VI weakness; gaze palsies; internuclear or complete ophthalmoplegia; and end-gaze nystagmus.16 Symptoms can fluctuate throughout the day, usually worsening as the day progresses or with prolonged muscle group use, such as with prolonged reading or prolonged chewing during a meal. Despite the presence of profound muscle weakness, there usually is no deficit in sensory, reflex, or cerebellar functioning. Myasthenia gravis in elderly patients can be misdiagnosed as ischemic stroke, especially when new-onset facial weakness is seen.17
Although weakness is typically focal and mild to moderate in severity, rarely, undiagnosed patients with myasthenia gravis may present with extreme weakness in the muscles of respiration, resulting in respiratory failure. This life-threatening situation, termed myasthenic crisis, can be seen before diagnosis or as a result of inadequate drug therapy or drug tolerance.
Consider the diagnosis of myasthenia gravis in any patient who complains specifically of ocular disturbances or proximal limb muscle weakness not associated with systemic causes of generalized fatigue. Involvement of the facial muscles or muscles of mastication and swallowing may suggest myasthenia gravis, as well as the observations that the symptoms fluctuate, often worsening as the day progresses, and are alleviated by rest.18 The differential diagnosis includes congenital myasthenia gravis, Lambert-Eaton syndrome (seen with small-cell lung tumors), drug-induced myasthenia (e.g., penicillamine, procainamide, quinines, aminoglycosides), botulism, thyroid disorders, and other causes of ocular disorders, such as intracranial mass lesions.
The diagnosis is established through the administration of edrophonium chloride (an acetylcholinesterase inhibitor); electromyography, which demonstrates a postsynaptic neuromuscular junctional dysfunction with repetitive nerve stimulation; and serologic testing for acetylcholine receptor antibodies. In the presence of abnormal neuromuscular transmission, edrophonium or neostigmine is expected to improve muscle strength in objectively weak limb, ocular, and pharyngeal muscles. Because these drugs can actually cause profound weakness in the presence of other disorders that impair neuromuscular transmission, be prepared to provide ventilatory support or perform endotracheal intubation as a complication of pharmacologic testing. Electromyographic testing with repetitive nerve stimulation demonstrates a rapid reduction in the size of the muscle action potential, a finding that correlates with the clinical observation of enhanced weakness with prolonged or repetitive muscle use.
Treatment includes administration of the acetylcholinesterase inhibitors pyridostigmine or neostigmine, thymectomy, chronic immune suppression with corticosteroids or azathioprine, and acute immune modulation using plasma exchange or IV immunoglobulin when indicated.19,20,21 A favorable response to thymectomy can occur in patients with a thymoma, a limited response to acetylcholinesterase inhibitor therapy, and a short time interval between diagnosis and operative intervention.22,23 Most patients show improvement with oral corticosteroids in the short term, although high-dose steroids sometimes result first in more weakness before improvement. Azathioprine or mycophenolate24 can supplement chronic oral steroid therapy and lowers the steroid dose.24,25 Severe symptoms, such as those that would require hospital admission, might require the use of IV immunoglobulin26,27 or a combination of high-dose steroids and plasma exchange.
Muscle weakness usually does not return to normal even with the use of immunomodulators. Variability in the amount of muscle weakness occurs in response to asthma exacerbations, infections, menstruation, pregnancy, emotional stress, hot weather, and other disorders that alter the response to medication, such as pulmonary, renal, and GI disease.
Many drugs used in the ED affect neuromuscular function—especially common antibiotics (Table 173-1). Make sure that myasthenia gravis patients being treated for other conditions receive their usual dose of cholinergic inhibitors, such as pyridostigmine, while waiting in the ED. The suggested pyridostigmine dose is 60 to 90 milligrams PO every 4 hours. If a dose is missed, the next dose is usually doubled. If the patient cannot take oral medications or is intubated, administer one-thirtieth of the PO dose (2 to 3 milligrams) of pyridostigmine by slow IV infusion. The usual IV dose for neostigmine is 0.5 milligram. Consult a neurologist to determine the optimal IV dose, rate of infusion, and timing of repeat pyridostigmine or neostigmine dosing.
| Steroids | Adrenocorticotropic hormone,* methylprednisolone,* prednisone* |
| Anticonvulsants | Phenytoin, ethosuximide, trimethadione, paraldehyde, magnesium sulfate, barbiturates, lithium |
| Antimalarials | Chloroquine,* quinine* |
| IV fluids | Sodium lactate solution |
| Antibiotics | Aminoglycosides, fluoroquinolones,* neomycin,* streptomycin,* kanamycin,* gentamicin, tobramycin, dihydrostreptomycin,* amikacin, polymyxin A, polymyxin B, sulfonamides, viomycin, colistimethate,* lincomycin, clindamycin, tetracycline, oxytetracycline, rolitetracycline, macrolides, metronidazole |
| Psychotropics | Chlorpromazine,* lithium carbonate,* amitriptyline, droperidol, haloperidol, imipramine |
| Antirheumatics | d-Penicillamine, colchicine, chloroquine |
| Cardiovascular | Quinidine,* procainamide,*β-blockers (propranolol, oxprenolol, practolol, pindolol, sotalol), lidocaine, trimethaphan; magnesium; calcium channel blockers (verapamil) |
| Local anesthetics | Lidocaine,* procaine,* |
| Analgesics | Narcotics (morphine, hydromorphone, codeine, Pantopon, meperidine) |
| Endocrine | Thyroid replacement* |
| Eye drops | Timolol,* echothiophate |
| Others | Amantadine, diphenhydramine, emetine, diuretics, muscle relaxants, central nervous system depressants, respiratory depressants, sedatives, procaine,* phenothiazines |
| Neuromuscular blocking agents | Tubocurarine, pancuronium, rocuronium, gallamine, dimethyl tubocurarine, succinylcholine, decamethonium |
The most significant ED complication of myasthenia gravis is respiratory failure, which is usually precipitated by infection, surgery, or the rapid tapering of immunosuppressive drugs. Although intubation should be considered in patients with a low forced vital capacity or in the presence of abnormal blood gas analysis, this decision is made primarily on clinical grounds. Because of the increased sensitivity of myasthenia gravis patients to neuromuscular junction inhibitors and an unpredicTable reaction to succinylcholine in particular,28 avoid the administration of depolarizing or nondepolarizing paralytic agents in preparation for intubation. Patients with myasthenia are extremely sensitive to these agents, and the paralytic effects can be expected to persist at least two to three times longer than in normal patients. Consider using short-acting agents such as etomidate, fentanyl, or propofol in smaller doses.29 Intubation using deep inhalational anesthetics, including halothane, isoflurane, or sevoflurane, is also a possible strategy. If paralytic agents are absolutely necessary, consider using one-half the dose of these agents, although there are no clinical studies supporting this recommendation.
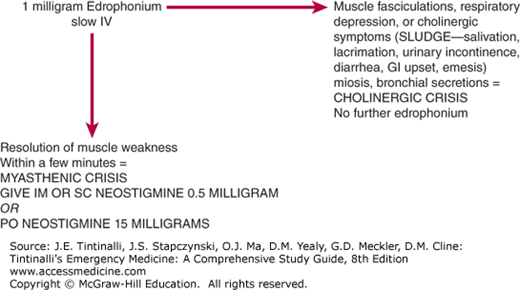
Up to 15% to 20% of myasthenia gravis patients will undergo a myasthenic crisis requiring acute emergency intervention.30 Myasthenic crisis, which occurs either because of disease exacerbation or inadequate drug therapy, must be distinguished from cholinergic crisis, which is caused by excessive cholinergic effects of the drugs used to treat the disease. This differentiation can be made in the ED by the use of edrophonium chloride testing (Table 173-2 and Figure 173-1). Edrophonium is used for this purpose because of its rapid onset (30 seconds) and the short duration of effects (5 to 10 minutes). A positive result, one that suggests that the symptoms are caused by a myasthenia exacerbation, is characterized by the resolution of muscle weakness within a few minutes. To ensure that the patient does not react adversely to the edrophonium test (as a result of excessive baseline cholinergic effects), administer only 1 milligram by slow IV push initially. The development of muscle fasciculations, respiratory depression, or cholinergic symptoms within a few minutes of this test dose of edrophonium suggests that the baseline muscle weakness is related to a cholinergic crisis, rather than a myasthenic crisis, and further edrophonium administration is contraindicated. If there is no evidence of adverse cholinergic effects, up to 10 milligrams of edrophonium can then be given in order to demonstrate benefit in the face of a presumed myasthenic crisis. In other words, if the symptoms improve with the 1-milligram edrophonium test dose, then the testing is considered positive for myasthenic crisis, and additional anticholinergic therapy can be provided to reverse the effects of myasthenia gravis itself. Neostigmine can then be given IM or SC in 0.5- to 2.0-milligram doses, with clinical effectiveness by 30 minutes and lasting for up to 4 hours. Alternatively, 15-milligram neostigmine tablets can be given PO, each having a clinical effect comparable with that of a 0.5-milligram parenteral neostigmine injection.
| Myasthenic Crisis | Cholinergic Crisis | |
|---|---|---|
| Pathology | Undermedication, decrease in acetylcholine receptor causes decreased stimulation by ACh | Overmedication, excess anticholinesterase drugs, overstimulation by ACh |
| Finding after edrophonium administration | Visible improvement in muscle contractibility, fusion of diplopia, or resolution of ptosis | Worsening of symptoms, muscle weakness, and possible respiratory paralysis |
| Implication of edrophonium test finding | Patient positive for myasthenia gravis, undermedication of anticholinesterase drugs | Overmedication has occurred, possibly due to insufficient effect from anticholinesterase drugs |
| Clinical treatment required based on test results | Increase in anticholinesterase drugs, such as pyridostigmine and neostigmine | Treat with atropine; if respiratory paralysis occurs, assist with ventilation |
In children, the total edrophonium IV dose is 0.15 milligram/kg, not to exceed 10 milligrams. To test adverse cholinergic effects with a test dose in children, give an initial IV edrophonium dose one tenth that of the total dose. For children weighing <75 lb (34 kg), a test dose of 1 milligram is appropriate, and a total dose of 5 milligrams can be used in 1-milligram increments. In infants, or when IV access is not available in children <75 lb (34 kg), the IM edrophonium test dose is 0.5 to 2.0 milligrams.
If giving edrophonium to patients with cardiac disease, place the patient on a cardiac monitor and pulse oximetry, have the crash cart with atropine available, and have intubation equipment prepared. Edrophonium may cause bradycardia, atrioventricular block, atrial fibrillation, and cardiac arrest. Although atropine will counteract the muscarinic effects (miosis, lacrimation, salivation, bradycardia) of edrophonium, it will not reverse the nicotinic effects (skeletal muscle paralysis) of a cholinergic crisis. Acute respiratory failure can result from either acute myasthenic crisis or acute cholinergic crisis. Patients with cholinergic crisis who worsen with edrophonium test dose administration may require immediate intubation and management of excessive secretions and acute bronchospasm.
When determining final disposition, consider other complications of muscle weakness in patients with myasthenia gravis, such as impaired swallowing, aspiration pneumonia, dehydration, and decubitus ulcers.
The American Academy of Neurology provides a guideline on medical treatment of the ocular symptoms involved in myasthenia gravis, as well as a summary of thymectomy indications.31 Additionally, the European Federation of Neurological Societies has formulated a versatile therapeutic plan for treatment.32 Cyclosporin and cyclophosphamide significantly improve myasthenia gravis, whereas azathioprine, mycophenolate, and tacrolimus have not been found to provide significant benefit.33 Corticosteroids are stated to be useful short-term.34
MULTIPLE SCLEROSIS
Multiple sclerosis (MS) is a neurologic disorder that causes variable motor, sensory, visual, and cerebellar dysfunction as a result of multifocal areas of CNS myelin destruction. Paresthesias, gait difficulty, extremity weakness, poor coordination, and vision disturbances occur most often with a relapsing and remitting clinical course. Despite the lack of a definitive cure, immunosuppression and immunomodulation provide adequate symptomatic relief in the majority of patients, such that most have only mild to moderate lifetime morbidity and a reduction in overall life expectancy of only 5 to 10 years.35
Three clinical courses are noted in patients with MS. Up to 90% have a relapsing and remitting course, with relapses lasting weeks to months. The remaining patients have either a relapsing and progressive course or a chronically progressive clinical course, the latter of which is more common with advanced age.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree