Patricia Lowry Coronary heart disease (CHD) remains the primary cause of death for both men and women in the United States. CHD includes acute myocardial infarction (MI), angina pectoris, atherosclerotic cardiovascular disease (ASCVD), and all forms of chronic ischemic heart disease. In 2011, 375,295 Americans died of CHD. Each year, an estimated 525,000, Americans have a new coronary attack (defined as first hospitalized MI or CHD death), and approximately 210,000,000 have a recurrent attack.1 It is estimated that an additional 155,000 silent first MIs occur each year. Approximately every 34 seconds one American has a coronary event, and approximately every 84 seconds an American will die of CHD.1 Disparities in the treatment of racial and ethnic minorities appear to be improving over time. In an assessment of a statewide program for treatment of ST-elevation myocardial infarction (STEMI), institution of a coordinated regional approach to triage and management was associated with significant improvements in treatment times that were similar for whites and blacks and for women and men. At least 68% of people older than 65 years with diabetes die of some form of heart disease. Heart disease death rates among adults with diabetes are two to four times higher than the rates for adults without diabetes. Diabetes mellitus is associated with higher short- and long-term mortality after STEMI, and in patients with diabetes mellitus both hyperglycemia and hypoglycemia are associated with worse outcomes. Myocardial tissue perfusion after restoration of epicardial coronary flow is more impaired in patients with diabetes mellitus. Early reperfusion for patients with an acute MI improves left ventricular (LV) systolic function and survival; therefore every effort must be made to minimize hospital delay. Although time is critical in the treatment of MI, it is patient delay, not transport or system inadequacy, that has proved to be the biggest obstacle to timely medical treatment. Half of patients experiencing STEMI do not seek medical care for approximately 1.5 to 2 hours after symptom onset, and one quarter delay seeking care for up to 6 hours. Little has changed in this time interval for the past 10 years, despite community-wide educational efforts to increase patient awareness of symptoms. Patient delay times are often longer in women, blacks, older adults, and Medicaid-only recipients and are shorter for Medicare recipients (compared with privately insured patients) and patients who are taken directly to the hospital by emergency medical services (EMS) transport. Patients may delay seeking care because their symptoms differ from their preexisting belief that a heart attack should manifest dramatically with severe, crushing chest pain. Approximately one third of patients with MI experience symptoms other than chest pain. Other reasons for delay in seeking treatment include (1) inappropriate reasoning that symptoms will be self-limited or are not serious; (2) attribution of symptoms to other preexisting conditions; (3) fear of embarrassment should symptoms turn out to be a “false alarm”; (4) reluctance to trouble others unless “really sick”; (5) preconceived stereotypes of who is at risk for a heart attack, an especially common trait among women; (6) lack of knowledge of the importance of rapid action, the benefits of calling EMS or 911, and the availability of reperfusion therapies; and (7) attempted self-treatment with prescription and/or nonprescription medications.2 To avoid such delays, health care providers should assist patients when possible in making anticipatory plans for timely recognition and response to an acute event.3 Family members, close friends, or advocates also should be enlisted as reinforcement for rapid action when the patient experiences symptoms of possible STEMI. Discussions should include a review of instructions for taking aspirin and nitroglycerin in response to chest pain. It is currently believed that it is the composition, morphology, and stability of the coronary artery plaque rather than the degree of plaque stenosis that determines the risk of cardiovascular events. Modification of controllable cardiac risk factors has been shown to decrease the frequency of cardiovascular morbidity and mortality. Historically, risk factors have been subdivided into factors that are nonmodifiable, such as gender, age, and family history, and factors that are modifiable, such as smoking cessation, dyslipidemia, diabetes mellitus, increased waist-to-hip ratio, physical inactivity, poor diet, psychosocial stress, and hypertension. There are specific recommendations to modify risk factors for patients who already have coronary artery disease (CAD) (Table 120-1). Studies suggest that 90% of the variability of acute MI can be attributed to modifiable risk factors. It is now known that some cardiac risk factors are more predictive of coronary artery events than others are. TABLE 120-1 AHA/ACC Secondary Prevention for Patients with Coronary Artery Disease and Other Atherosclerotic Vascular Disease* Lifestyle modifications, including adherence to a heart-healthy diet, regular exercise habits, avoidance of tobacco products, and maintenance of a healthy weight, remain an important component of health promotion and ASCVD risk reduction. Lipid lowering with the use of statins has also been supported for the primary prevention of ASCVD in many higher-risk individuals and for secondary prevention in all individuals. In 2013 the American College of Cardiology (ACC) and American Heart Association (AHA) released new guidelines on the treatment of blood cholesterol.4 Four statin benefit groups have now been identified and include individuals in need of primary or secondary prevention. The first group includes those in need of secondary prevention who have demonstrated clinical ASCVD. This is defined by an acute coronary syndrome (ACS), history of MI, stable or unstable angina, coronary or other arterial revascularization, stroke, transient ischemic attack (TIA), or peripheral vascular disease (PVD). The other three categories involve patients in need of primary prevention. The first of these categories involves those with elevations of low-density lipoprotein cholesterol (LDL-C) above 190 mg/dL without a secondary cause such as high saturated fats or causative drugs. The next category involves those primary prevention patients with diabetes aged 40 to 75 years with LDL-C of 70 to 189 mg/dL. The last category involves those without diabetes aged 40 to 75 years with LDL-C of 70 to 189 mg/dL and an estimated 10-year ASCVD risk score5 higher than 7.5%. The new guidelines base the intensity of statin therapy to reduce ASCVD risk according to those most likely to benefit (Table 120-2). There are therefore no more low-density lipoprotein (LDL) treatment targets. For those individuals unable to tolerate high-intensity statin therapy, moderate-intensity statin therapy is recommended. Nonstatin therapies (e.g., niacin, fibrates, ezetimibe), whether alone or in addition to statins, were not found to provide acceptable ASCVD risk reduction benefits and should be avoided.6 TABLE 120-2 High-, Moderate-, and Low-Intensity Statin Therapy Chronic stable angina is precipitated by exertion and relieved by rest. A reduction in myocardial oxygen supply or increases in myocardial oxygen demand are the determinants of coronary ischemia. Although the pathologic process for unstable angina and the pathologic process for chronic stable angina both result from atherosclerotic lesions in the coronary arteries, the pathophysiologic mechanism of each varies. Under normal circumstances, an increase in myocardial oxygen demand is balanced by an increase in myocardial oxygen supply. The three most important factors that determine myocardial oxygen demand are heart rate, systemic blood pressure (peripheral vascular resistance), and LV wall tension. The heart rate and the systolic blood pressure exert independent influence on myocardial oxygen requirements because both determine myocardial workload (heart rate × systolic blood pressure = myocardial workload). Therefore, activities (e.g., exercise, hurrying, lifting) and increased metabolic demands (e.g., with fever, anemia, thyrotoxicosis) that increase the workload of the heart in the presence of a fixed and limited oxygen supply will increase myocardial oxygen requirements and thus precipitate ischemia and angina. The coronary arteries exhibit changes in vascular tone (vasomotion). These changes play a significant role in the development of coronary ischemia. Under normal circumstances, the endothelial or innermost lining of the coronary artery responds to vasoactive stimuli, such as mental stress, cold, and catecholamines, by releasing endothelium-derived relaxing factor (EDRF) to maintain vasodilation. In the presence of atherosclerosis, however, the endothelial function is impaired; hence, the vasoconstrictive response is unopposed, leading to constriction at the site of atherosclerosis and adjacent areas. This results in a decrease in myocardial blood flow and induces coronary ischemia. It has been recognized that asymptomatic occurrences of ischemia are more common than symptomatic episodes in patients with exertional angina symptoms. Silent myocardial ischemia occurs when there is objective evidence of ischemia in the absence of symptoms. Since the advent of continuous ambulatory electrocardiographic monitoring, many patients with typical stable angina have been found to have frequent episodes of asymptomatic ischemia.7 The full clinical implications of silent ischemia are not well understood, but there is increased incidence of ischemia, MI, and sudden death in asymptomatic patients with positive exercise stress test results. In addition, patients with asymptomatic ischemia who have had an MI are at greater risk for a second coronary event. Ischemia can occur with or without evidence of increased myocardial oxygen demand (increased product of heart rate and blood pressure). Diabetic patients are at a twofold to fourfold greater risk of cardiovascular mortality compared with those without diabetes, and silent myocardial ischemia on stress testing occurs more often in patients with diabetes than in patients without diabetes. The pathogenesis of silent myocardial ischemia is not well understood, although several hypotheses exist. It has been suggested that some individuals have a higher endorphin level than others do, which may play a role in the perception of pain. In addition, some patients have a higher ischemic pain threshold and greater tolerance of cold-induced ischemia. Finally, autonomic dysfunction, particularly in patients with diabetes, is thought to contribute to silent ischemia. The cause of microvascular angina is still not fully understood, although studies have demonstrated that some patients with this syndrome have an abnormal vasodilating response of their small or resistance vessels (diminished coronary reserve). Still other patients may have a low pain threshold or other noncardiac causes of pain. Most patients with microvascular angina have cardiac risk factors such as family history of heart disease, hypertension, tobacco abuse, diabetes, hyperlipidemia, and abdominal obesity. Women seem to be more prone to this than men, especially those with decreased estrogen levels. The diagnosis of microvascular angina (syndrome X) is suspected when there is a convincing history of anginal chest pain with or without documented reversible ischemic electrocardiographic changes, angiography fails to demonstrate obstruction or spasm of a major coronary artery, and other conditions have been excluded from the differential diagnosis. In variant angina, coronary artery spasm should be suspected on the basis of the patient’s history. Spasm can occur in any coronary artery; however, the right coronary artery and, to a lesser extent, the left anterior descending artery are more commonly affected. The spasm tends to be focal and reproducible at the same location. However, diffuse single-vessel coronary artery spasm may occur. Multivessel spasm is extremely rare; when it occurs, it is associated with intractable ventricular tachycardia. The cause of coronary artery spasm is abnormal endothelial cell function. This is especially true when injury to the endothelium results in decreased concentration of EDRF. The pathophysiologic mechanism of acute MI has been controversial since Hippocrates first postulated that heart disease could cause sudden death. The causes of MI can be divided into those that decrease myocardial oxygen supply and those that increase myocardial oxygen demand. Atherosclerotic plaque results in a reduction of coronary blood flow, thereby reducing oxygen supply. These plaques reduce the cross-sectional area of the coronary artery lumen, thus reducing coronary perfusion pressure. When a critical stenosis develops, coronary blood flow is adequate at rest but cannot increase to meet metabolic demands during exertion. The development of a vulnerable coronary artery lesion is multifactorial and depends on the biochemical and physical properties of that lesion. Unstable angina can be divided into those that decrease myocardial oxygen supply and those that increase myocardial oxygen demand by a nonocclusive thrombus that has developed from a ruptured atherosclerotic plaque. Plaque rupture initiates an inflammatory response, which stimulates chemotactic factors for circulating monocytes. Monocytes enter the vessel wall, transform into tissue macrophages, and ingest oxidized LDLs. Over time, lipid-filled macrophages (foam cells) die, creating an extracellular lipid pool with eventual formation of a fibrous cap. Proteolytic enzymes produced by activated macrophages erode the fibrous cap, producing areas that are fragile and prone to rupture. Increases in shear stress and vasomotor changes placed on this vulnerable lesion make it highly likely to rupture. Plaque rupture causes vessel damage and initiates platelet activity. Platelet aggregation and activation develop a platelet-rich “white clot” over the endothelial damage, causing partial obstruction of flow in the artery and thus unstable angina and non–ST-segment elevation myocardial infarction (NSTEMI). Therefore the role of the inflammatory response as a trigger for plaque rupture cannot be overemphasized. Bacterial and viral infections make plaques more vulnerable and unstable with a predisposition to rupture and thrombose. A number of studies have shown the benefit of evaluating high-sensitivity C-reactive protein to determine cardiovascular risk.8 When plaque rupture occurs, the size of the resultant thrombus, whether it is a small mural thrombus or an occlusive thrombus, depends on several factors, including the amount of thrombogenic substrate that is exposed, the amount of local blood flow disturbance, and the actual thrombotic propensity of the vessel. Therefore, lesion disruption is a dynamic process that may lead to transient vessel occlusion and ischemia by a labile thrombus, resulting in unstable angina. These thrombotic occlusions often resolve spontaneously; however, they can recur within hours or days. In other cases, formation of a fixed thrombus and a more chronic occlusion may occur, resulting in acute MI. Coronary artery narrowing of less than 80% typically does not induce development of collateral vessels. For this reason, smaller plaques that rupture are more likely to cause a significant clinical event during thrombotic occlusion of the vessel as a result of the absence of protective collateral flow. In most cases, MI occurs when an atherosclerotic plaque ruptures, which serves as a nidus for thrombus formation with resultant coronary artery occlusion. The atherosclerotic plaque most likely to rupture is the nonocclusive plaque, which may rupture several times before MI is produced. With each rupture, blood, fibrin, and platelet aggregates accumulate in the plaque, forming intraintimal or intraplaque thrombus and resulting in increases in plaque size, intraplaque pressure, and obstruction of the coronary lumen. When such a plaque ruptures, fissures, or ulcerates, MI or sudden death may occur. Plaque rupture with resulting thrombus formation is the common physiologic mechanism underlying unstable angina, MI, and sudden death. The amount of myocardial injury sustained is directly related to several factors, including the amount of thrombus present, the ability of the intrinsic lytic system to promote lysis, the impact of local vasoconstrictor substances on impeding blood flow, whether the vessel affected is partially or totally occluded, the presence or absence of collateral vessels and the quantity of blood they supply to the affected area, and the amount of myocardium supplied by the affected vessel. The platelet is not only the smallest cell but also the most active in thrombus formation. The platelet consists of membranes, tubules, granules, and receptors. During activation, the resting platelet undergoes a dramatic change that induces platelet-platelet interaction or aggregates. Such platelet aggregates play an important role in ACS and MI. Patients who died of unstable angina, MI, and sudden cardiac death have platelet aggregation, fibrin, and microthrombi as common findings. Because platelets are important in the pathophysiologic process of acute ischemic syndrome and MI, inhibition of platelet activation should be beneficial in reducing and preventing ACS. Myocardial infarction with nonobstructed coronary arteries (MINOCA) is an emerging syndrome. Cardiac registries’ prevalence measurements indicate that 10% of patients with diagnosed MI do not have obstructions in their coronary arteries.9 In 2015, researchers used PubMed and Embase to conduct a meta-analysis of studies mentioning nonobstructed coronary arteries in the setting of MI. For this review, these researchers defined MINOCA as documented presence of MI with angiographic findings of less than 50% stenosis in any epicardial artery. Findings from this meta-analysis indicate that MINOCA is a finding in 6% of MI patients, confers a better 12-month mortality prognosis than in documented CAD (prognosis for patients with MINOCA is still guarded), and has structural dysfunction, coronary spasm, and thrombotic disorders found in association.9 Current recommendations are to consider MINOCA as a working diagnosis while evaluating these patients for treatable underlying causes with magnetic resonance imaging (MRI), provocative testing, and evaluation for thrombophilia.9 The patient with chronic stable angina demonstrates characteristic symptoms that occur with predictable frequency, severity, duration, and provocation. These symptoms occur with exertion, are relieved by rest or no more than one nitroglycerin tablet, and in general last for only 1 to 3 minutes. Chronic stable angina remains constant unless an acceleration of the disease process intervenes. The clinical presentation can best be evaluated by a detailed history of angina quality, location, radiation, severity, duration, and precipitating and relieving factors. Associative factors such as dyspnea, diaphoresis, nausea, vomiting, eructations, diarrhea, and fatigue should also be evaluated (Box 120-1). William Heberden first defined the peculiar discomfort of myocardial ischemia as angina pectoris, which translated means “strangling in the chest.” The majority of patients do not refer to their angina symptoms as pain; thus, questioning related to “chest pain” may prove misleading, and the diagnosis of angina pectoris may be missed. Discomfort originating in the chest may arise from many structures, including the skin, subcutaneous tissue, bone, muscle, vascular structures, nerves, pleura, lungs, pericardium, heart, esophagus, and gastrointestinal viscera. Adjectives used to describe the quality of angina can be variable; it is often conveyed as a pressure, heaviness, aching, constriction, tightness, squeezing, numbness, or burning sensation. Patients may demonstrate a clenched fist over the sternal area (Levine sign) to further elucidate this feeling. The location of discomfort is predominantly behind the midsternum (retrosternal) or just to the left of the sternum, in an area approximately the size of a clenched fist. If the patient is able to localize the area of discomfort as being no larger than a fingertip, the sensation is seldom related to myocardial ischemia, and other causes should be considered. Myocardial ischemia can also encompass the territory between the epigastrium and the lower jaw, lower teeth, and hard palate, with sensations of tightness or constriction in the throat area. Atypical symptoms are more common in women, the elderly, and diabetic patients. Radiation symptoms are not uncommon and are related to involvement of the C8 to T4 spinal ganglia. These ganglia receive impulses from the heart and from peripheral dermatomes that are transmitted to the spinal cord through afferent nerve fibers. When myocardial ischemia occurs, the sharing of these ganglia can produce discomfort to the other dermatomal areas. Thus, stimulation of the dermatomes affecting the brachial plexus can result in discomfort or numbness anywhere along the medial surface of the left arm, including the fourth and fifth digits. Isolated wrist discomfort has also been reported. The right arm and lateral surfaces can be affected, although with less frequency. Stimulation of the cervical plexus can result in suprascapular and intrascapular discomfort. Precipitating factors, including increased exertion, coitus, and emotion, tend to induce myocardial ischemia by increasing circulating catecholamine levels. This increases the metabolic oxygen needs of the heart in the setting of a limited oxygen supply, thereby producing angina symptoms. Eating of a large meal may precipitate discomfort, as can the increased metabolic demands from fever, chills, thyrotoxicosis, anemia, hypoglycemia, exposure to cold air, and the nicotine from cigarette smoking. Relief of stable angina symptoms generally occurs within 1 to 3 minutes after the discontinuation of activity or with rest. When angina is related to emotional upheaval, it may take longer for catecholamine levels to decrease, and angina symptoms may persist for a longer period. Nitroglycerin administration will usually provide relief within 5 minutes and is a useful diagnostic tool. When symptoms persist for longer than 20 minutes, the patient should no longer be considered to be having chronic stable angina and should be instructed to seek prompt medical attention. Although cessation of activity generally produces relief of pain, it has been noted that some patients who develop angina with walking are able to continue walking, with eventual alleviation of the angina. These patients are able to “walk through” the angina event. There are several proposed hypotheses for the relief of angina during exercise. These include dilation of functioning collateral blood vessels during exercise; relief of coronary arterial spasm; and vasodilation of systemic blood vessels with a corresponding decline in systemic arterial blood pressure and heart rate, which in turn reduces myocardial oxygen demand. The Canadian Cardiovascular Society classification (CCSC) is a useful tool to determine the exercise tolerance of patients with stable angina pectoris and to determine the degree of disability that angina symptoms are imposing on the patient (Box 120-2). This simple instrument can be used to risk stratify and to manage patients with angina.10 Myocardial ischemia can be experienced as dyspnea, indigestion, nausea, numbness in the upper extremities, and fatigue rather than actual chest pressure; this is particularly true in women.11 Symptoms of dyspnea are generally noted to be stable when they occur with moderate exertion and unstable when they occur with minimum exertion or with rest or when they begin to awaken the patient during the night. The cause of stable symptoms is related to increased myocardial demand, and the cause of unstable symptoms is related to decreased myocardial supply. The dyspnea produced is caused by myocardial ischemia resulting in diastolic dysfunction, which produces increased left-sided filling pressures. Fatigue often follows an activity and resolves within several minutes. The cause is related to LV dysfunction resulting in decreased cardiac output. The clinical presentation for microvascular angina is chest pain, which is often unpredictable and may occur with rest, routine physical activity, or stressful events. Unlike chest pain from stable angina, chest discomfort from microvascular disease is generally more intense, lasts for longer periods of time, and does not go away with rest. Discomfort is generally not responsive to nitroglycerin. Although there is no apparent gender difference in the perception of angina, the syndrome of microvascular angina is found predominantly in women. The sine qua non of variant angina pectoris is a history of spontaneous or unprovoked episodes of typical angina. Discomfort occurs predominantly at rest and is usually not provoked by exertion. Patients sometimes note that beta blockers exacerbate symptoms. The differential diagnosis on presentation should be unstable angina until it is proven otherwise. Diagnosis of unstable angina and NSTEMI depends predominantly on a detailed patient history. The most important factors from the initial history that enhance the likelihood of the patient’s experiencing an episode of ischemia are the nature of symptoms, prior history of CAD, age older than 65 years, and number of risk factors present for CAD. The Thrombolysis in Myocardial Infarction (TIMI) risk score is one of several valued tools for use in the emergency department for risk stratification and therapeutic decision-making (Table 120-3).12 The risk score encompasses these factors and adds electrocardiographic findings and cardiac marker data as well as aspirin use in the previous 7 days. The use of aspirin and concomitant angina was found to be a powerful predictor of unstable angina or NSTEMI. In addition, several factors may suggest an acceleration of the patient’s chronic angina symptoms to unstable angina or NSTEMI. These factors may include occurrence of the angina event with less provocation or at rest, prolongation of the angina symptoms, increase in the severity of symptoms, and newly associated findings with the chest discomfort. Physical examination findings of pulmonary edema, new or worsening mitral regurgitation murmur, S3 heart sound, hypotension, bradycardia, or tachycardia suggest that the patient is at high risk. According to guidelines, a 12-lead electrocardiogram (ECG), preferably with and without chest pain, should also be obtained. It is particularly important to assess the duration of angina events and whether rest pain has been present to determine the patient’s short-term risk of complications. Patients who develop NSTEMI have a 70% higher risk of death and an 8.5% higher potential for reinfarction than do those with unstable angina alone.13 TABLE 120-3 TIMI Risk Score for Patients with Unstable Angina and NSTEMI: Predictor Variables Classically, acute MI is diagnosed as a constellation of symptoms. Chest pain described as pressure, heaviness, squeezing, crushing, and aching is often associated with nausea, vomiting, diaphoresis, or dyspnea. In general, the pain involves the sternum or epigastrium; in many cases, it may radiate to the arm, elbow, jaw, or neck. Any combination of these symptoms may occur in an individual patient. Epigastrium pain secondary to acute MI may be misdiagnosed as indigestion, and referred pain to the shoulder on deep inspiration may be misdiagnosed as being splenic in nature. In the older patient, MI may manifest as a sudden onset of dyspnea, weakness, loss of consciousness, or confusion. Although chest discomfort may be the most common presenting symptom, it may be atypical or absent in some patients with ACS (silent acute MI). Inspection of the chest may reveal the point of maximum impulse (PMI) to be downward or laterally displaced, suggestive of cardiomegaly, perhaps from hypertension. The PMI may also have a rocking quality, perhaps related to a LV aneurysm from a previous MI. The thorax should be inspected to determine the presence of any rashes or vesicles, which may suggest a herpetic cause of the discomfort. Inspection of the neck veins should be performed to assess the jugular venous pulse for any elevation. The contour of the internal jugular waveforms should also be noted. A funduscopic examination may reflect hypertension or diabetic retinopathy. Xanthomas or an early arcus senilis may be indicative of elevated cholesterol levels. The peripheral circulation should be assessed for any vascular lesions suggestive of arterial or venous disease. Palpation during cardiac assessment is confined to assessment of the upstroke of the carotid artery pulse and the PMI of the cardiac apex. The carotid upstroke should be of normal volume and intensity. A prolonged carotid upstroke may indicate aortic stenosis because ventricular emptying becomes delayed when it is ejected across a significantly stenotic valve. Conversely, a brisk carotid upstroke may indicate aortic regurgitation or hypertrophic cardiomyopathy. The PMI should be confined to the fifth intercostal space at the midclavicular line. With any downward or lateral displacement of the PMI, cardiomegaly should be considered. In a follow-up inspection, palpation of the PMI should confirm any aneurysm formation. Auscultation of the chest may reveal a ventricular gallop (S3) produced just after the second heart sound, which may be either physiologic or pathologic in nature. A physiologic S3 may be heard in children and adults up to 35 to 40 years old. It may also be noted in women during their third trimester of pregnancy. A pathologic S3 may be related to decreased myocardial contractility and is suggestive of heart failure caused by volume overload of the ventricles. This may be related to either mitral or tricuspid regurgitation. An atrial gallop (S4) may be noted just before the first heart sound and is produced by an increased resistance to ventricular filling caused by ventricular stiffness after atrial contraction. LV causes of an S4 include cardiomyopathy, hypertension, MI, and aortic stenosis. Right ventricular (RV) causes include pulmonary hypertension and pulmonary stenosis. An S4 may also be noted in trained athletes. A pansystolic murmur audible at the apex during an episode of chest pain is most likely consistent with mitral regurgitation. It is often secondary to papillary muscle dysfunction as a result of LV ischemia. A ventricular septal defect after MI should also be considered and further evaluated with echocardiography. Inflammation around the pericardium may produce a pericardial friction rub, which generally has one systolic and two diastolic components. The systolic component is produced when the ventricles contract in systole, whereas the diastolic components are produced in early and late diastole. The early diastolic component is a result of rapid, passive ventricular filling, whereas the late diastolic component occurs with atrial contraction. The sound produced is very high and of a scratching or grating quality. Adventitious breath sounds suggest heart failure. Their occurrence and the presence of any vascular bruits, indicating further vascular disease, should prompt further evaluation. The physical examination findings are usually normal when the patient is not having episodes of variant angina; however, during episodes, the patient may develop hypertension and tachycardia in response to the pain. In addition, the patient may have associated diaphoresis, nausea, and radiation of pain to the arm. Auscultation of the chest during an episode may reveal a gallop or transient systolic murmur originating from the mitral valve. Approximately 90% of the diagnosis of an acute coronary event is made from the patient’s history, ECGs and laboratory data. The physical examination findings will support this diagnosis and help determine whether the patient is in heart failure or is manifesting evidence of a cardiac arrhythmia. The patient will understandably be anxious and on occasion will be diaphoretic. The pulse rate and blood pressure may be normal; however, with an extensive area of MI, the patient may have a compensatory tachycardia and be hypotensive (Box 120-3).
Chest Pain and Coronary Artery Disease
Definition and Epidemiology
Patient-Related Delays and Initial Treatment
![]() Immediate emergency department referral or physician consultation is indicated for patients with suspected MI.
Immediate emergency department referral or physician consultation is indicated for patients with suspected MI.
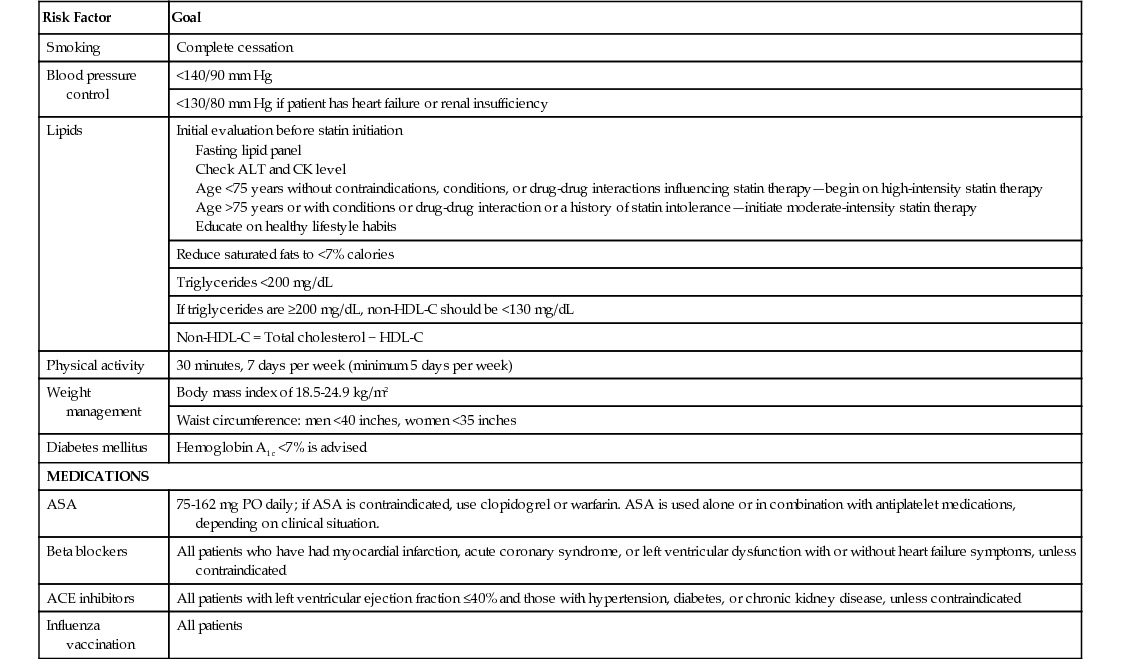
Risk Factors for Coronary Artery Disease
Risk Factor
Goal
Smoking
Complete cessation
Blood pressure control
<140/90 mm Hg
<130/80 mm Hg if patient has heart failure or renal insufficiency
Lipids
Initial evaluation before statin initiation
Fasting lipid panel
Check ALT and CK level
Age <75 years without contraindications, conditions, or drug-drug interactions influencing statin therapy—begin on high-intensity statin therapy
Age >75 years or with conditions or drug-drug interaction or a history of statin intolerance—initiate moderate-intensity statin therapy
Educate on healthy lifestyle habits
Reduce saturated fats to <7% calories
Triglycerides <200 mg/dL
If triglycerides are ≥200 mg/dL, non-HDL-C should be <130 mg/dL
Non-HDL-C = Total cholesterol − HDL-C
Physical activity
30 minutes, 7 days per week (minimum 5 days per week)
Weight management
Body mass index of 18.5-24.9 kg/m2
Waist circumference: men <40 inches, women <35 inches
Diabetes mellitus
Hemoglobin A1c <7% is advised
MEDICATIONS
ASA
75-162 mg PO daily; if ASA is contraindicated, use clopidogrel or warfarin. ASA is used alone or in combination with antiplatelet medications, depending on clinical situation.
Beta blockers
All patients who have had myocardial infarction, acute coronary syndrome, or left ventricular dysfunction with or without heart failure symptoms, unless contraindicated
ACE inhibitors
All patients with left ventricular ejection fraction ≤40% and those with hypertension, diabetes, or chronic kidney disease, unless contraindicated
Influenza vaccination
All patients
Lipid Guidelines
High-Intensity Statin Therapy
Moderate-Intensity Statin Therapy
Low-Intensity Statin Therapy
Daily dose lowers LDL-C on average by approximately >50%
Daily dose lowers LDL-C on average by approximately 30% to <50%
Daily dose lowers LDL-C on average by 30%
Atorvastatin 40-80 mg
Rosuvastatin 20-40 mg
Atorvastatin 10-20 mg
Rosuvastatin 5-10 mg
Simvastatin 20-40 mg
Pravastatin 40-80 mg
Lovastatin 40 mg
Fluvastatin 40 mg bid
Simvastatin 10 mg
Pravastatin 10-20 mg
Lovastatin 20 mg
Pathophysiology
Chronic Stable Angina
Silent Myocardial Ischemia (Asymptomatic Coronary Heart Disease)
Microvascular Angina (Syndrome X)
Variant Angina (Coronary Artery Spasm, Prinzmetal Angina)
Unstable Angina and Non–ST-Segment Elevation Myocardial Infarction
Acute ST-Segment Elevation Myocardial Infarction
MINOCA: Suspected Myocardial Infarction with Nonobstructed Coronary Arteries
Clinical Presentation
Chronic Stable Angina
Anginal Equivalents
Microvascular Angina
Variant Angina
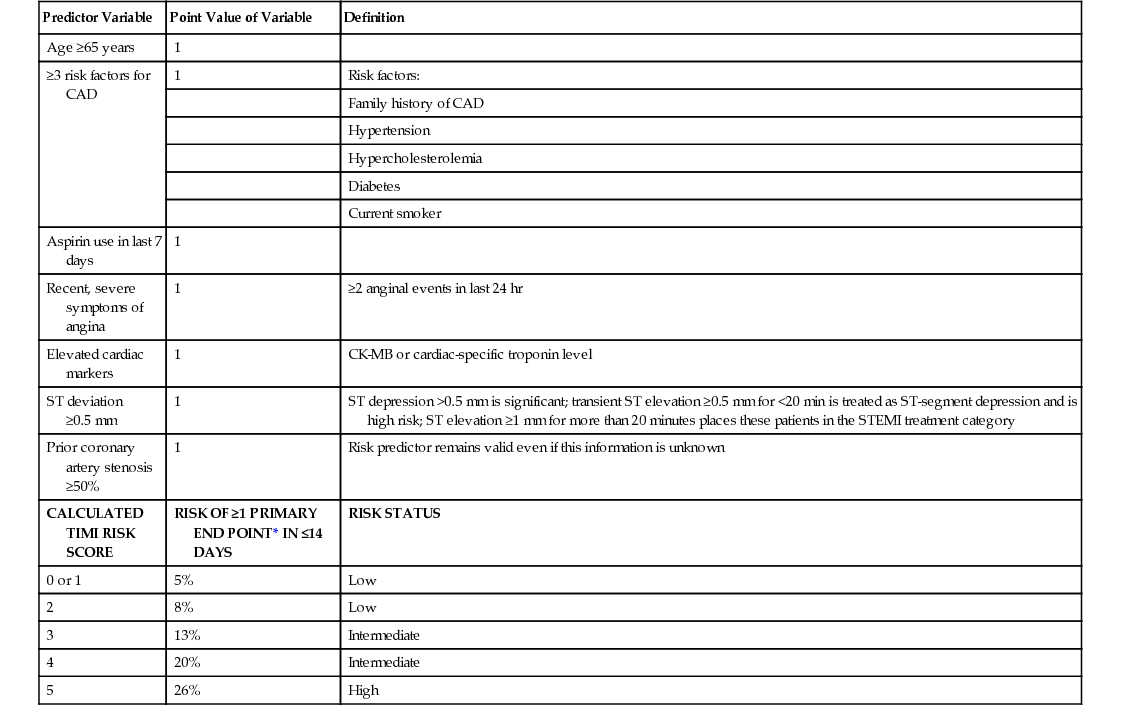
Unstable Angina and Non–ST-Segment Elevation Myocardial Infarction
Predictor Variable
Point Value of Variable
Definition
Age ≥65 years
1
≥3 risk factors for CAD
1
Risk factors:
Family history of CAD
Hypertension
Hypercholesterolemia
Diabetes
Current smoker
Aspirin use in last 7 days
1
Recent, severe symptoms of angina
1
≥2 anginal events in last 24 hr
Elevated cardiac markers
1
CK-MB or cardiac-specific troponin level
ST deviation ≥0.5 mm
1
ST depression >0.5 mm is significant; transient ST elevation ≥0.5 mm for <20 min is treated as ST-segment depression and is high risk; ST elevation ≥1 mm for more than 20 minutes places these patients in the STEMI treatment category
Prior coronary artery stenosis ≥50%
1
Risk predictor remains valid even if this information is unknown
CALCULATED TIMI RISK SCORE
RISK OF ≥1 PRIMARY END POINT* IN ≤14 DAYS
RISK STATUS
0 or 1
5%
Low
2
8%
Low
3
13%
Intermediate
4
20%
Intermediate
5
26%
High
Acute ST-Segment Elevation Myocardial Infarction
Physical Examination
Chest Pain and Coronary Artery Disease
Chapter 120







