Clinical Vignette
A 47-year-old Japanese man new to your office arrives complaining of fatigability, lassitude, poor memory, irritability, and hypervigilance whenever he has to go to public places. As part of your standard social history, you learn that he was formerly a maintenance worker in the Tokyo subway system before emigrating to the United States in 1996. He tells you he was present during the Aum Shinrikyo attack in 1995. At that time, he experienced eye irritation, rhinorrhea, wheezing, cramps, nausea, and diarrhea. He was treated overnight at a local hospital. He has never felt the same and wonders if his current problems are related to the sarin gas attack. What do you tell him?
Background
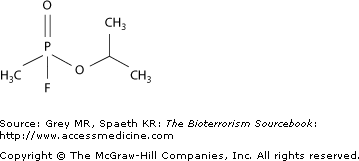
In 1936, German scientist Gerhard Schrader discovered tabun, the first in a class of chemical weapons known as nerve agents. The discovery of a compound with similar effects, sarin, was made in 1938 (Fig. 25–1). Sarin would come into worldwide notoriety in the terrorist attack on the Tokyo underground system in March 1995 (Fig. 25–2). Nerve agents are compounds classified as organophosphates (OPs) which were originally developed for use as pesticides. The code names GA, GB, and GD, representing tabun, sarin, and soman, respectively, were standardized by the Tripartite pact after WWII. The “G” in these code names simply stands for German. Rapid analysis and trials of antidotes for these agents were begun as the Cold War loomed, though Soviet production of nerve agents began as early as 1946. As a practical matter, clinicians are far more likely to encounter occupational or environmental organophosphate poisoning than nerve agent exposure from terrorist incidents (Table 25–1). However, the knowledge gleaned is generalizable to their use as weapons.
| June 1994. Aum Shinrikyo releases sarin gas in Matsumoto, exposing over 300 persons, hospitalizing 56, and killing 7. First responders and health care workers also develop mild symptoms of nerve gas exposure. |
| March 1995. The same terrorist group releases sarin gas in the Tokyo subway, affecting 5,000 to 6,000 riders, hospitalizing nearly 500, killing 12, and causing permanent neurologic deficits in many. |
| Sarin gas is part of both the U.S. and Russian chemical weapons stockpiles, with 5,000 tons and 11.7 tons, respectively. |
| Countries suspected of stockpiling nerve agents: India, South Korea, Iraq, Syria, Egypt, Iran, Libya, and North Korea. |
| Saddam Hussein launched 2 major and 280 smaller chemical weapons attacks on the Kurds of northern Iraq during the 1980s using mustard gas and sarin gas. |
| Russian military used “knockout gas” (fentanyl) to end a hostage situation in a Moscow theater, killing 115 hostages and incapacitating 50 Chechnyan terrorists. |
Properties
Nerve agents exist as liquids at room temperature and are usually diluted in an aqueous solution. The liquids vary in color from colorless to brown. Although several of these agents have odors described as “fruity,” odor is an unreliable means of identifying most chemicals in either an occupational or a bioterrorist context. The hydrophilicity and lipophilicity of nerve agents are responsible for their rapid absorption through the skin, mucous membranes, and clothing.
Volatility and biopersistence varies among the different categories of nerve agents. G agents exhibit less biopersistence compared to V agents (Table 25–2). Chemical modification or alterations in carrier agents may affect volatility and biopersistence as well. Thickening agents, such as acrylates, increase viscosity and thus prolong biopersistence. They also add to the risk of secondary aerosolization, given the right environmental conditions. All nerve agents are rapidly inactivated by strong alkalis and chlorines, a fact that has implications for clinical management and decontamination (see Decontamination and Treatment). Nerve agent vapors are designed to be denser than air, and if undisturbed by wind, vapors remain close to the ground. This property imposes greater risk of exposure to children or to adults in low-lying spots. Vaporization of nerve agents occurs when heat from an explosive device disperses its vaporization of droplets aerosolized by a sprayer. Secondary vaporization can occur from the ground or from other surfaces that have been coated with the liquid or droplets.
| Odor | Biopersistence | Aging Time | |
|---|---|---|---|
| G Agents | |||
| Cyclohexyl sarin (GF) | Odorless | Persistent | >4 hr |
| Sarin (GB) | Odorless | Persistent | 3–4 hr |
| Soman (GD) | Fruity odor | Persistent | 2 min |
| Tabun (GA) | Fruity odor | Persistent | >4 hr |
| V Agents | |||
| VX | Odorless | Nonpersistent | >40 hr |
Of Note . . .
After several failed attempts to release botulinum toxin in a Japanese government building, the worldwide religious cult based in Japan, Aum Shinrikyo, which preaches an apocalyptic message and whose name means Supreme Truth, levied a successful attack.
On June 27, 1994, members of the sect drove a truck into a suburban neighborhood of Matsumoto, Japan, where three of the judges presiding over a lawsuit against the sect were being housed. They released sarin gas, which reached a housing complex, killing 7, hospitalizing 200, and sending 500 people in seek of medical attention.
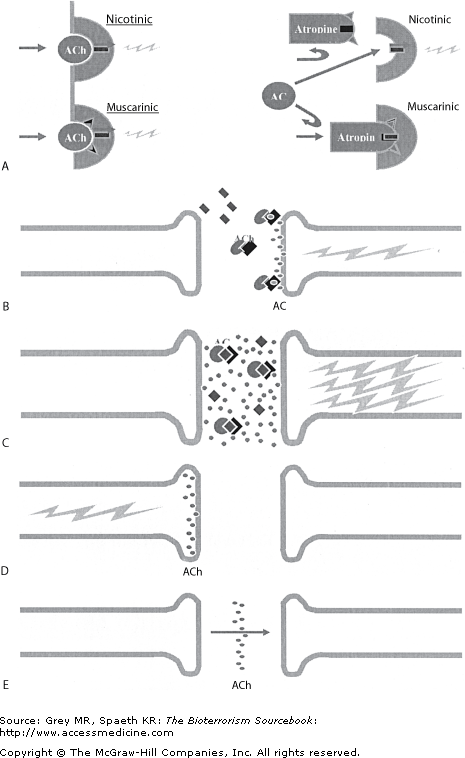
On March 20, 1995, Aum Shinrikyo members placed packages containing sarin gas in five different trains of the Tokyo subway system, one of the five trains service many key governmental agencies. Riders first noticed the odor of solvents and acute eye irritation, but for some this was soon followed by dyspnea, muscle weakness, and unconsciousness. Most affected individuals managed to escape the train before succumbing. Nicotinic symptoms of pallor, tachycardia, hypertension, muscle fasciculation, and muscular weakness predominated, with less prominent muscarinic effects (e.g., sweating, secretions, and bradycardia). More severely affected individuals experienced seizures. Within hours it was over: somewhere between 5,000 and 6,000 commuters were affected. Over 550 individuals were transported by ambulance, and nearly 3,000 more managed to find their own way to one of Tokyo’s 41 hospitals, area urgent care facilities, or private physician offices. Four hundred ninety-three were admitted to hospitals. Seventeen required intensive care, and 12 died. The majority of those presenting for evaluation had mild symptoms and were sent home. The majority of those admitted were observed and discharged within 48 hours. All told, 12 Japanese commuters died, most within the first 24 hours. Two individuals died 2 weeks later of hypoxic brain injury, and others experienced permanent residual neurologic deficits. One hundred thirty-five ambulance drivers, police, and health care workers also developed symptoms, and 33 were hospitalized. Calls flooded Tokyo’s telephone system, making it nonfunctional for hours following the attack.
When investigators located and raided the site of sarin production, they found a crude production facility capable of producing very large quantities of chemical weapons using readily available equipment and under the supervision of cult members who were themselves scientists. Evidence showed that Aum Shinrikyo was attempting to develop biological weapons, including botulinum toxin, anthrax, cholera, and Q fever. They even made efforts to acquire Ebola virus. Although arrests and greater surveillance has damaged the group, they continue to recruit members and spread its apocalyptic message.
Sources
Short of industrial or military sabotage, or a chemical terror event, only those individuals involved in the production, transportation, or storage of nerve agents are likely to be at risk for inadvertent exposure to nerve agents. Production, storage, and transportation practices are similar to those involved with organophosphate pesticides, and individuals require full hazmat gear and work in highly controlled settings with engineering equipment and PPE to limit inadvertent exposure and industrial accidents. Storage facilities must be vigilant regarding security measures.
Pathophysiology
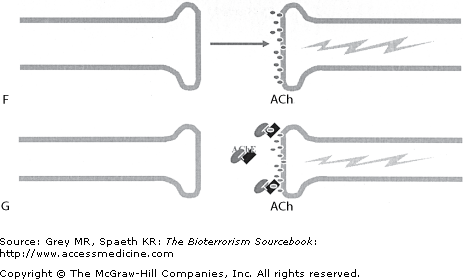
Nerve agents’ physiologic and clinical effects mirror those of the organophosphate family of pesticides. They act by irreversibly inactivating esterase enzymes, the most clinically relevant one being acetylcholinesterase (AChE). AChE acts in the synaptic cleft throughout the peripheral nervous system, as well as in the autonomic nervous system and central nervous system (CNS). Its function is to degrade the neurotransmitter acetylcholine (ACh). Nerve agents bind covalently to AChE within seconds or minutes, causing structural changes that render the enzyme useless. After the formation of the covalent bond, an isopropyl (alkyl) group detaches from the enzyme as part of a process referred to as “aging.” Once aging occurs, the enzyme is irreversibly impaired. The loss of enzymatic breakdown results in the accumulation of ACh in the synapse and hyperstimulation of muscarinic and nicotinic receptors throughout the body. This hyperactivity, as well as the direct disruption of neurons in the CNS, define the clinical presentation of nerve agents. Although inhibition of AChE is responsible for the clinical syndrome, inhibition of other esterase enzymes is of clinical value in diagnosing exposure to either organophosphates or nerve agents. Specifically, both serum and red blood cell (RBC) cholinesterase levels are useful as markers of exposure to organophosphates and nerve agents.
Toxicity
The extent and severity of the consequences from an exposure are dependent on the particular nerve agent used. VX is by far the most potent of the nerve agents currently known to exist (Table 25–3).
There are two primary means of exposure. The G agents are vapors under most conditions and so are inhaled or absorbed through the conjunctiva, whereas the V agents are liquids and are absorbed through the skin. Ingestion is a possible but unlikely route of exposure. Inhalational exposure is most likely to occur when nerve agents are released in an aerosolized form or if sprayed (Fig. 25–3), such as from a helicopter or crop duster. Systemic absorption occurs within minutes. The rapidity with which clinical effects occur is dose and time dependent. In general, vapor exposure elicits symptoms immediately or within minutes, with peaks occuring within a few minutes after exposure. Vapor can also be absorbed through the eyes concurrent to respiratory absorption. Percutaneous exposure occurs when liquid forms of the nerve agents come into contact with the skin, eyes, mouth, or membranes of the nose (Fig. 25–4).
Aside from being the most potent of the nerve agents (note the LD50), VX is also the least volatile of the agents, thereby requiring smaller amounts of absorption for effects to occur. Conversely, sarin is far more volatile and is more likely to evaporate before absorption through the skin can take place.
Clothing or other barriers to the skin reduce exposure, depending on the nature (material, layering, etc.) of the barrier. An important exception is wet cloth, which can increase exposure and minimize evaporation. Temperature plays a role in the rate at which nerve agents are absorbed: absorption increases as temperatures increase. Absorption through the skin yields signs and symptoms within minutes, but may take as long as thirty minutes. With lesser exposure, symptom onset may be many hours and will typically involve GI features. A rule of thumb is that the longer the delay in symptom onset, the less severe the clinical course.
Signs and Symptoms
Broadly speaking, the presentation of signs and symptoms for nerve agents may be divided into local and systemic effects. If the exposure level is small, then local symptoms may be all that are present. For example, a very small dermal exposure might present with localized muscle fasciculations and sweating. Far more likely is that there will be sufficient exposure to result in systemic symptoms. The route of exposure dictates whether systemic or local effects are seen.
Local Symptoms and Signs: Vapor Exposure
Nerve agents in vapor form result in exposure and absorption through the respiratory tract and eyes. Symptom onset occurs almost immediately and may last up to forty-eight hours. By the time a patient is seen in an office or hospital setting after exposure to vapor, it is quite likely the peak effect has occurred and no new symptoms should be expected.
It is important to note that ophthalmic findings can occur strictly as a local response and are not, of themselves, proof of systemic effects. When the eyes are exposed to nerve agent vapor, miosis, conjunctival hyperemia, eye pain, and a frontal lobe headache will be noted within a few minutes of exposure. With a minimal exposure, the signs and symptoms will likely resolve within twenty-four hours. With more intense exposures, signs and symptoms may last up to seventy-two hours.
Absorption through the respiratory tract represents the most efficacious means of absorption. Within a few minutes, rhinorrhea, wheezing, chest tightening, and nasal hyperemia occur. If the exposure is relatively limited, these symptoms should end within several hours after exposure. As with the eyes, it is important to note that upper respiratory tract symptoms can occur without systemic symptoms.
Liquid Exposure
Large liquid exposures result in the onset of symptoms within half an hour, although it is possible for symptoms to be delayed for as long as a day after exposure. The shorter the time until onset, the more severe the clinical features. Should exposure to nerve agents occur by contact with liquid forms, signs would be noted focally with profuse sweating and fasciculations at the site of contact within a couple of minutes to hours and last upward of five days. If liquid nerve agents come in contact with the eyes, the signs and symptoms and their respective onset and duration is the same as those listed for vapor exposure to the eye. The only significant difference is that the onset occurs instantaneously. If liquid nerve agents are ingested, the signs and symptoms are similar to the systemic affects as discussed later, though, not surprisingly, the first organ system to be affected will be the GI tract.
Systemic Symptoms and Signs
The ubiquity of muscarinic, nicotinic, and cholinergic receptors throughout the body explains the wide range of symptoms and signs experienced by individuals exposed to nerve agents. Substantial variability in clinical presentation is common, however, and driven by host factors (e.g., genetic receptor heterogeneity or co-morbidities) and environmental factors (e.g., route of exposure and dose). Due to the clinical variety adopting a syndromic diagnostic approach is useful; a comprehensive list is provided (Table 25–4), although selected clinical syndromes are discussed in more detail. Children may not show the characteristic miosis and hypersecretion of adults and may present with more of the neurologic symptoms and signs.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree