Clinical Vignette
A 40-year-old Kurdish émigré presents to your office accompanied by his daughter who translates for him. As a teenager, his village was gassed by the Iraqi military. He developed diffuse skin erythema over most of his body, profound eye pain and photophobia, and blepharospasm. No blisters developed, but within several hours he noticed mild upper airway irritation and a dry cough. He did not have any shortness of breath. Within a few days, he noticed his skin became dark and some areas desquamated. In the following six months he had recurrent respiratory and skin infections, but he has been otherwise well for the past twenty-four years. His exam is significant for patchy areas of hyperpigmentation.
There are two major classes of vesicants: the mustards and the arsenicals. Each class is discussed using a prototypical agent. A third agent, often classified with the vesicants, is phosgene oxime. This compound is poorly understood, but a brief discussion is warranted and can be found at the end of this chapter. Vesicants are more “strategic” in so far as they tend to injure rather than kill (compared to other classes of chemical weapons.) They cause injury by blistering whatever part of the body is exposed—internal or external. They may be released via bombs or aerosolized through sprays.
Sulfur Mustards
Mustards were identified in the early to mid-1800s, while arsenicals were developed in the United States during WWI. Although first used in 1917, three years into WWI and three years after the military use of chlorine gas began, mustards resulted in more WWI casualties than all the other chemical weapons combined—accounting for upward of 80% of all chemical agent causalities. Vesicants have been used since WWI, most notably by Iraq during the 1980s. The Iran–Iraq war inflicted an estimated 50,000 casualties, mostly against Kurdish Iraqis in northern Iraq. At present, no antidote exists for mustard gas. This is unfortunate as nearly a dozen countries maintain mustard as part of their cadre of chemical agents. In contrast to their use as chemical weapons, members of this class have found use as chemotherapy for certain types of cancers.
Mustards, including the nitrogen and sulfur types, are thought to be named for the pungent odor or from the yellow-brown color. They are among the most persistent of the chemical agents. Historically and militarily, sulfur mustards have played a bigger role and are felt to be more of a threat as a weapon of terror. Nitrogen mustards are considered battlefield agents and so are less likely to be encountered by the public (Fig. 24–1), but are discussed briefly as well.
The chemical structure of mustard results in a highly reactive sulfur moiety, which may account for its toxicity (Fig. 24–2). Despite having a characteristic smell, odor is not felt to be a reliable means of detecting mustards. Mustards are oily liquids at room temperature because of their high boiling point, but as ambient temperatures rise, vapors can form. Mustard vapor can linger for days and, because of its density, it accumulates in low-lying areas. The LD50 for liquid mustard is approximately 1.5 teaspoons, enough liquid to cover up to 20% of the body. Mustard vapor’s LCt50 is considerably lower and represents a more potent risk of injury than many other chemical agents (Table 24–1). Mustards are vulnerable to water, which dissolves this class of compounds rendering them harmless; the warmer the water, the faster the rate of destruction.
| Properties | Impure Sulfur Mustard (H) | Distilled Sulfur Mustard (HD) | Phosgene Oxime (CX) | Lewisite (L) |
|---|---|---|---|---|
| Chemical and Physical | ||||
| Boiling Point | Varies | 227°C | 128°C | 190°C |
| Vapor Pressure | Depends on purity | 0.072 mmHg at 20°C | 11.2 mmHg at 25°C (solid) | 0.39 mmHg at 20°C |
| 13 mmHg at 40°C (liquid) | ||||
| Density: | ||||
| Vapor | Approx 5.5 | 5.4 | <3.9? | 7.1 |
| Liquid | Approx 1.24 g/mL at 25°C | 1.27 g/mL at 20°C | ND | 1.89 g/mL at 20°C |
| Solid | NA | Crystal: 1.37 g/mL at 20°C | NA | NA |
| Volatility | Approx 920 mg/m3 at 25°C | 610 mg/m3 at 20°C | 1,800 mg/m3 at 20°C | 4,480 mg/m3 at 20°C |
| Appearance | Pale yellow to dark brown liquid | Pale yellow to dark brown liquid | Colorless, crystalline solid or a liquid | Pure: colorless, oily liquid |
| As agent: amber to dark brown liquid | ||||
| Odor | Garlic or mustard | Garlic or mustard | Intense, irritating | Geranium |
| Solubility: | ||||
| In Water | 0.092 g/100 g at 22°C | 0.092 g/100 g at 22°C | 70% | Slight |
| In Other Solvents | Complete in CCl4, acetone, other organic solvents | Complete in CCl4, acetone, other organic solvents | Very soluble in most organic solvents | Soluble in all common organic solvents |
| Environmental and Biological | ||||
| Detection | Liquid: M8 paper | Liquid: M8 paper | M256A1 ticket or card | Vapor, M256A1 ticket or card, ICAD |
| Vapor: CAM | Vapor: CAM, M256A1 kit, ICAD | |||
| Persistence: | ||||
| In Soil | Persistent | 2 wk? 3 yr | 2 h | Days |
| On Material | Temperature-dependent; hours to days | Temperature-dependent; hours to days | Nonpersistent | Temperature-dependent; hours to days |
| Skin | M2581 kit | |||
| Decontamination |
|
| Water |
|
| Biologically Effective Amount: | ||||
| Vapor (mg? min/m3) | LCt50: 1,500 |
|
| Eye: <30 |
| Skin: approx 200 | ||||
| LCt50: 1,200–1,500 (inhaled) 100,000 (masked) | ||||
| Liquid | LD50: approx 100 mg/kg | LD50: 100 mg/kg | No estimate | 40–50 mg/kg |
| Onset of Pain: | Hours later | Hours later | Immediate | Immediate |
| Onset of Tissue Damage | Immediate; onset of clinical effects is hours later; fluid-filled blister | Immediate; onset of clinical effects is hours later; fluid-filled blister | Seconds; solid wheal | Seconds to minutes; fluid-filled blister |
Commercial production of mustards does not occur in the United States. Individuals involved in securing, transporting, or destroying existing military stockpiles may be exposed accidentally. Exposed individuals may inadvertently carry the oily compound home on contaminated clothing, although this is unlikely. Individuals involved in laboratory and toxicologic testing of mustards represent another potential at-risk group. The general public may be exposed to sulfur mustard at hazardous waste sites that contain or are involved in the destruction of sulfur mustard. Mustards do not occur in nature. Water contamination is unlikely as mustard is rapidly hydrolyzed and rendered harmless.
It is not known with certainty how mustard causes cellular damage. This partly explains why no effective therapy exists. Two theories presently exist regarding the mechanism of injury. The most commonly accepted theory is that mustards act by creating reactive intermediary compounds that alkylate DNA, RNA, proteins, and other macromolecules in the cell, thereby inhibiting normal glycolysis, breaking DNA strands, and activating apoptosis. A second hypothesis is that mustard consumes intracellular glutathione, one of the prime cellular defense mechanisms against free radicals. Such injury leads to a loss of homeostatic mechanisms—calcium regulation and membrane integrity, in particular. Soon thereafter, cell death occurs. These agents are referred to as radiomimetic because they mimic the clinical effects of ionizing radiation. Though both theories have some basis in animal studies, neither sufficiently explains the histopathologic changes seen in mustard victims. What is certain is that mustard has its greatest insult on rapidly dividing cells (hence, the therapeutic role as chemotherapy). The effects occur within a few minutes of tissue contact, and its presence in the body quickly becomes undetectable. Such rapid reactivity and elimination is important to keep in mind because it means that blood, skin, blisters, or blister fluid are not contamination risks to those aiding the victims.
The skin blistering caused by mustards is thought to result from two effects: damage to the dermal proteins that anchor the epidermal layer and liquefaction necrosis of the deeper cell layers of the skin. The fluid within the blister is a consequence of an inflammatory response to the cellular damage and does not contain mustard itself.
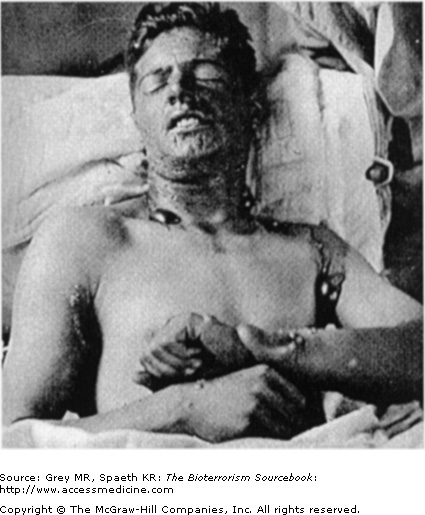
The organ systems affected by mustards are those that come into direct contact with the liquid or vapor: such as the eyes, skin, or respiratory system (Table 24–2). More severe exposures may result in hematologic, nervous, and gastrointestinal toxicity. A distinctive clinical aspect of mustard gas exposure is a latent period before the onset of injury. Tissue damage can occur without immediate symptoms of burning, pain, or itching. As a result, exposed but asymptomatic individuals may not take necessary avoidance or decontamination steps. Only the mustard agents are initially pain free—arsenicals and phosgene oxime (both discussed later) are painful immediately.
| Pattern Identification | Multiple, concurrent cases of erythematous, blistering skin |
| Features | Skin—spectrum from erythema to bullae |
| Eyes—conjunctivitis, ophthalmitis, lacrimation, irritation | |
| Respiratory—cough, hoarseness, SOB, ARDS | |
| GI—nausea, vomiting | |
| Findings | Blistered skin, pulmonic involvement, rhinitis, lacrimation |
| Mild to moderate exposure—leukocytosis | |
| Severe exposure—pancytopenia. ARDS, death |
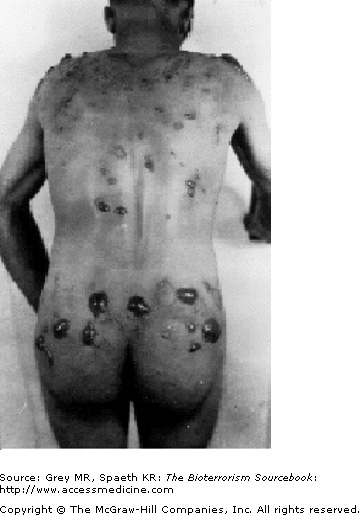
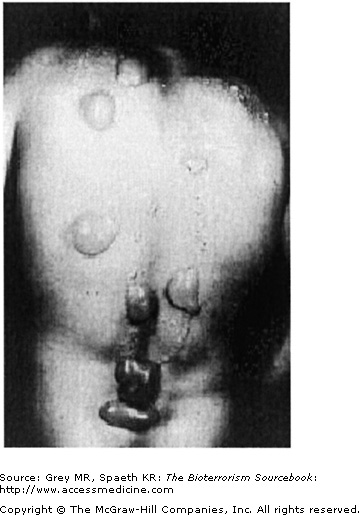
Sites most prone to injury are those that tend to be warm and moist: neck, antecubital fosse, and perineum. With mild exposure, skin erythema, similar to that seen with sunburn occurs and is associated with burning or itching. The area may be mildly edematous as well. More commonly, skin exposure results in the formation of blisters that begins with the formation of multiple, pinpoint vesicles on the periphery of the site that coalesce into bullae, ranging in size from 0.5 to 5.0 cm and usually sitting on an erythematous base. The wall of the blisters are typically thin and translucent and contain fluid that initially is clear but later turns yellow and coagulates. Mustard agents are not present in the blister fluid. The extent and severity of the skin injury is a consequence of the intensity of exposure and of the skin sensitivity of the individual. Severe exposure may cause centrally necrotic lesions (Fig. 24–3). Mustard vapor is less toxic to the skin and more likely to result in first- or second-degree burns, whereas liquid mustard is more likely to cause severe skin injury, that is, third-degree burns, and often develops quickly (Fig. 24–4).
As noted previously, mustard’s clinical impact occurs after a latency period lasting from a few hours to a day. A distinguishing feature is that during this latency period despite cellular insult, the patient feels little pain and indeed may be altogether unaware of exposure. This lack of awareness may prevent the exposed individual from leaving a contaminated area or taking appropriate decontamination procedures. The latency period is followed by the emerging skin erythema, and, if blisters follow they often tear, or become encrusted (Fig. 24–5). Reepithelization occurs as it would after any kind of burn, with erythema resolving within a few days and uncomplicated blisters (i.e., without secondary infections) resolving within a month. As with other burns, the extent of skin involvement determines mortality rates. Burns and blisters covering more than 25% of the body are associated with higher mortality rates.