Epidemiology
The agents in this section are typically diseases of animals; with the exception of psittacosis, the animals are usually livestock, although other animals may be carriers. Psittacosis is commonly viewed as an avian disease (it is also known as ornithosis). Typhus fever is the only one of the group carried and passed by an invertebrate. As is characteristic of Category B diseases, these agents do not carry a high mortality rate. In fact, many infections with Category B agents will resolve without medical intervention. Typhus fever has the highest mortality rate of the group, roughly 50% if untreated, but death is rare with treatment. With Category B agents the health concerns are more with debility and psychological consequences, rather than with death or permanent or serious injury. Clinicians and public health officials are likely to be familiar with many of these agents in their natural or sporadic form, but they have potential as low-tech biological weapons and are therefore included in the Sourcebook.
Infectious Agents: Q Fever (Coxiella burnetii)
The “Q” in Q fever stands for “query” because investigators looking into one of the earliest documented outbreaks of the disease in the 1930s could not identify the causative agent. Later it was determined that the etiologic agent of Q fever was Coxiella burnetii, a rickettsial bacterium. Livestock, such as cattle and sheep, as well as other animals (e.g., cats and dogs) serve as natural reservoirs for the disease. Infected animals are typically asymptomatic.
There are reports that in the past the Soviet Union attempted to weaponize C. burnetii; whether this was successful is unknown. As a biological weapon, C. burnetii is most likely to be aerosolized, but it has potential as a food or waterborne contaminant. Q-fever is a self-limited illness, its primary impact as a bioterror weapon is to cause morbidity and spread fear.
C. burnetii has a hardy spore form that is capable of surviving for long periods outside the host, even with exposure to heat or dehydration. The spore form also tolerates many ordinary disinfectants. This agent is not highly pathogenic, is rarely fatal, and usually resolves even without treatment of any kind. It is highly infective, however. Indeed, some experts believe that even a single organism is sufficient to cause the illness. With infection, C. burnetii is found in higher concentrations in the products of gestation including placenta, amniotic fluid, as well as in the animal’s waste products.
C. burnetii is found throughout the globe and is considered a potential bioweapon primarily because of its impressive infectivity. Livestock and other animals are the primary hosts and therefore the source for cross-infection of humans living in close proximity to these animals, or having direct contact with the animals.
Although Q fever is found worldwide, its exact incidence is difficult to establish because so many cases go unreported due to the relatively mild clinical syndrome it causes. Mortality rates are very low; no more than 2% of those infected die as a result of the disease. This probably reflects underlying host factors, such as co-morbidities, immunosuppression, or the extremes of age.
Infection with Q fever occurs following inhalation of contaminated dust, consumption of infected animal products, such as milk or meat, or bites from ticks carrying the disease. As a biological weapon, dissemination as an aerosol is the most probable method for spreading the disease. It follows that inhalation is the primary route of exposure.
C. burnetii is an obligate intracellular parasite. Once inside the body, it is ingested by phagocytic cells where, in turn, the organism takes up residence in intracellular lysosomes. At this point, the bacterium does not cause injury to the cell or host organism. Once bacterial replication within the lysosome reaches a maximum load cell, cell lysis occurs. At this point, the infection triggers symptoms secondary to the host’s immune response. C. burnetii induces a biphasic antigenic response. Acutely, IgM antibodies predominate. In the chronic form, both IgG and IgM antibodies are demonstrable.
Following an incubation period of fourteen to twenty-one days, approximately half of all people infected with C. burnetii evince signs of clinical illness. Signs and symptoms tend to be nonspecific and, for the most part, benign. Most commonly, illness begins with sudden onset of fevers (up to 104–105°F) lasting up to fourteen days. This febrile phase is associated with severe headache, general malaise, myalgia, confusion, sore throat, chills, sweats, nonproductive cough, nausea, vomiting, diarrhea, and abdominal pain. Up to half of all patients will develop chest x-ray findings of pneumonia—with or without cough. Cough when present can be productive or dry, and pleuritic chest pain may occur as well. Approximately one-third of patients will develop acute hepatitis or signs of hepatic injury.
In most cases, signs and symptoms of infection resolve fully within a few weeks, even without treatment. Resolution may be delayed for as long as several months in some circumstances. Infection confers lifelong immunity.
Chronic forms of Q fever are rare but do exist. Infection may reemerge even after twenty years following an initial infection. Clinical manifestations of chronic or recurrent Q fever include chronic hepatitis, bacterial-negative endocarditis (seen most commonly in the presence of synthetic valves), encephalitis, and osteomyelitis. Chronicity carries a significantly higher mortality rate, upward of 70% in some clinical case series.
Mild elevations of liver enzymes, leukocytosis, and thrombocytopenia are laboratory findings seen with Q fever infection. In acute infection, IgM followed by IgG antibodies are present. In chronic disease, IgG antibodies predominate.
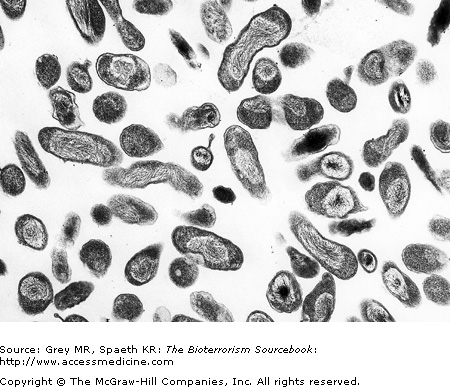
C. burnetii bacteria are pleomorphic bacilli or cocci that are difficult to discern on standard microscopy because they are intracellular. They are more readily identified with special stains such as Giemsa or using electron microscopy (Fig. 20–1).
Chest radiographic findings are not consistently seen even with respiratory symptoms such as cough and pleuritic pain. Conversely, radiographic abnormalities may be noted even in the absence of respiratory symptoms. When present, findings are consistent with pneumonia: a unilateral or occasionally multilobar infiltrate. Consolidation and effusions are less common.
Q fever is included in the differential diagnosis of flulike bioterror syndromes, including as well as the Category A agents Yersinia pestis and Franciscella tularensis. The differential diagnosis is expansive, including atypical bacterial pneumonias (e.g., Legionella or Mycoplasma) or influenza-like illnesses. See Table 20–2 for a list of other possible causes.
Nearly all untreated patients will recover; however, antibiotic therapy appears to serve a dual function: shortening the duration of the natural course as well as reducing the likelihood of recurrent disease. Drugs of choice for Q fever are tetracyclines, specifically doxycycline. Macrolides have been used empirically with good success and quinolones have shown efficacy in vitro as well. The optimal duration of therapy is less clearly defined but has the greatest impact on morbidity if started within 3 days of symptoms. The Centers for Disease Control and Prevention (CDC) recommends doxycycline 100 mg po twice a day for 14 to 21 days, whereas military sources cite a shorter 2 to 7 day course as adequate. Chronic or recurrent Q fever is treated with prolonged combination treatment with doxycycline and a quinolone. The duration of treatment for chronic infections varies depending on the source: the CDC recommends four years; U.S. military texts recommend two years.
A fully protective vaccine for Q fever exists. Presently, it is made in Australia and is not commercially available in the United States. Postexposure prophylaxis with doxycycline 100 mg orally twice a day for 5 to 7 days is appropriate if the patient demonstrates clinical symptoms or signs. Starting antibiotic prophylaxis during the incubation period, that is, prior to the onset of signs and symptoms is thought only to prolong the incubation period.
Brucellosis (Brucella species)
Background
One of the world’s most significant agents of animal disease, Brucella species are found naturally in a wide range of animal reservoirs including livestock, farm animals, and dogs. Brucellosis—also known as undulant fever due to its characteristic biphasic fever curve—is caused by a number of different species within the genus. Four of those are known to be infectious to humans. Because it is both an animal and a human disease, Brucella species can be wielded to inflict both human health and economic injuries. As an agent of bioterror it would most likely be used in aerosol form.
Properties
In its natural form, Brucellosis is highly infective, but as with Q fever it is not very virulent: it is debilitating but rarely fatal to those infected. Brucella species do not sporulate; nonetheless, they are quite hardy. The bacterium is able to survive for weeks in water or soil and resists environmental stresses such as cold temperatures. It does not survive desiccation caused by hot weather or direct exposure to sunlight.
Sources
Brucella species can be found indigenously in much of the world. Infected animals serve as naturally occurring sources via contaminated animal products, such as dairy or meat products, or by inhaling contaminated dust. As a result of diligent veterinary practices—specifically vaccination—most U.S. livestock are free of the disease. The primary natural reservoirs are bison and elk. Since the 1940s, several nations have sought to weaponize Brucella species, but whether these efforts were successful is unknown. There are no confirmed examples where Brucella has been used as a biological weapon.
Epidemiology
Means of Transmission
Transmission occurs as a result of ingestion of contaminated animal products such as unpasteurized milk or cheese, or from meat. It can also occur as a result of inhalation of contaminated dust or soil particulates as well as through direct contact of contaminated matter with skin openings, such as skin abrasions. Entry into the body occurs through epidermal breaks, the respiratory tract, the gastrointestinal tract, or the conjunctiva. Person-to-person transmission is extremely rare and documented only through breast-feeding and unsafe sexual practices. As a biological weapon, aerosolization of brucellosis and subsequent respiratory disease due to inhalation of the bacterium is the primary concern.
Pathogenesis
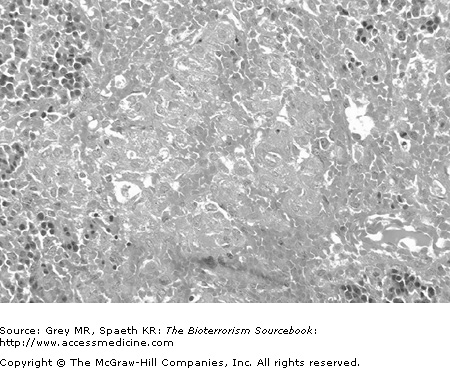
After finding its way into the body, Brucella is endocytosed by phagocytic macrophages. Brucella seems able to inhibit normal lysosomal activity within the macrophage and so is not destroyed by the intrinsic enzymatic pathways. Macrophages deliver the bacteria to local and regional lymph nodes where intracellular proliferation occurs until the lymphatic cells burst. The infection then spreads hematogenously throughout the body, being taken up preferentially by the reticuloendothelial system (liver, spleen, bone marrow, and lymph nodes). Less commonly, infection of the central nervous, cardiovascular, and renal systems may result from hematogenous dissemination. Granuloma formation is noted characteristically at tissue sites where infection is found, particularly in the liver and large lower axial joints (Fig. 20–3).
Signs and Symptoms
The clinical manifestations of brucellosis in the acute and chronic forms are nonspecific. Onset of signs and symptoms in the acute form may be immediate or insidious and occur following a 2 to 4 week incubation period. Documentation of incubation periods as long as two months underscores the potential difficulty in identifying deliberate biological attack with Brucella. Because of its predilection for the reticuloendothelial and lymphatic systems, symptoms of brucellosis may be localized or systemic. Symptoms include malaise, fatigue, myalgias, and headache. CNS symptoms such as depression and difficulty concentrating are reported commonly. Signs are few, with fever and chills being the most consistent clinical findings. Brucella gets its moniker of “undulating fever” from the diurnal pattern of the disease’s fever curve. Hepatosplenomegaly and lymphadenopathy are also found with some frequency. Complications of brucellosis include pancytopenia, axial joint arthritis, and tenosynovitis (commonly sacroiliitis), as well as orchiditis and epididymitis. Within the lung, brucellosis may cause interstitial pneumonia and pleural effusions, including empyema. Symptoms may last up to a month before resolving. Rare, but serious complications of brucellosis occur in fewer than 5% of cases, including encephalitis, meningitis, or endocarditis. These are responsible for the majority of brucellosis fatalities.
Chronic Effects
There is a chronic form of brucellosis characterized by symptoms that relapse every 2 to 3 weeks for upwards of a year and presents similarly to chronic fatigue syndrome. A deep or walled-off source of infection that seeds the body intermittently is thought to be the cause. Sequelae after infection are also quite variable and include granulomatous hepatitis, distal arthritis, anemia, leukopenia, thrombocytopenia, meningitis, uveitis, optic neuritis, papilledema, and endocarditis.
Laboratory Tests
Microscopy
Radiographic Findings
Differential Diagnosis
The nonspecific clinical manifestations of brucellosis make the differential diagnoses quite broad. Any chronic febrile illness, particularly one in which the fever relapses and remits, must include brucellosis. Syndromic surveillance categorization would places it in the flu-like category, along with the Category A agents plague, tularemia, and the Category B agent, Q fever. The differential diagnoses for brucellosis is found in Table 20–4.
Diagnosis
Treatment
Even without medical intervention, the majority of patients will recover from Brucella infection. Antibiotics reduce the course and severity, as well as the likelihood of recurrence and sequelae. Doxycycline 200 mg orally each day plus Rifampin 600 mg orally per day for 6 weeks is the CDC recommended regimen (See Appendix).
Vaccine
Infection Control
Person-to-person transmission does not occur, so isolation is not necessary; standard precautions will suffice. Contaminated objects are easily sterilized or disinfected by common disinfecting agents, such as phenol- or formalin-based solutions. Pasteurization is effective for treatment of contaminated dairy products.
Of Note . . .
In early 1999, a 38-year-old New Hampshire woman was admitted to a community hospital and eventually ended up on mechanical ventilation. Three weeks into her hospital stay she was diagnosed with brucellosis. She had no risk factors for the disease, such as animal contacts, camping, or recent travel history. Her family came forward with petri dishes, flasks, growth media and Brucella cultures belonging to her live-in boyfriend. The man was a foreign national and claimed to be a marine biologist who had been working at a local university but had recently returned to his country of origin. The woman eventually died of adult respiratory distress syndrome. The FBI and CDC investigated the matter, and no explanation of how she acquired the infection was ever found. Bioterrorism was not ruled out as having played a role.
Glanders (Burkholderia mallei)
Background
Glanders is a disease primarily affecting members of the equid family, including horses, mules, and donkeys. Other animals—humans included—are also susceptible to the disease. Human cases of glanders occur sporadically. In all likelihood, glanders has been used as a biological weapon for nearly a century. In World War I, the German army was suspected of having intentionally infected a large number of horses of the Russian army along the eastern front with this zoonotic disease. The Japanese infected horses, civilians, and prisoners of war with glanders (and other biological warfare agents) during its occupation of China. During World War II, unconfirmed reports suggest that the Soviet army used glanders during its occupation of Afghanistan in the 1980s. Excepting the biowarfare examples noted, there are no documented naturally occurring epidemics of the disease. As a biological weapon, aerosolized glanders represents the most concerning possibility.
Sources
Horses, mules, donkeys, goats, and domesticated animals, such as dogs and cats, are the natural reservoirs for Burkholderia mallei. Unlike its close relative Burkholderia pseudomallei (see next section), it is not found in environmental reservoirs such as water, soil, or plants. Laboratorians, veterinarians, outdoorsmen, and others with close physical contact with animal reservoirs, animal products, or microbial sources for research are at greatest risk of acquiring the disease in its sporadic form.
Properties
Epidemiology
Means of Transmission
How the bacteria spreads is not well understood. Epidermal breaks due to lacerations or other lesions are believed to be one method of transmission following direct contact with infected animals: entry through mucosal surfaces of the upper airway or on the conjunctiva appear to be another way. Infections among laboratorians working with B. mallei have demonstrated that aerosol infection is possible as well. In the context of biological weapons, the latter is the most likely means of transmission.
Pathogenesis
The virulence of B. mallei is due to compounds synthesized by the bacteria that are toxic to the host’s metabolism. The toxins tend to disrupt enzyme activity at key metabolic pathways. For example, the blue-green pigment pyocyanin, disrupts oxidative phosphorylation. Lecithinase, another of the toxins elaborated by the bacterium, causes cell lysis by degrading lecithin contained within cell membranes, collagenases, lipases, and hemolysins. If the production of these compounds reaches a certain threshold, clinical symptoms ensue. Because the symptoms result from toxins, antibiotics are not especially helpful in treating this disease.
Signs and Symptoms
There are three acute forms of glanders: focal, pulmonary, and septic. They may occur in isolation or in combination. A chronic form of the disease, known as “farcy,” presents with lymphangitis and lymphadenopathy. The focal form is seen following exposure through an epidermal break and direct bacterial contact after an incubation period of about five days. Following implantation, a nodular lesion with local lymphangitis and lymphadenopathy results. Similarly, infection of an upper respiratory site or ocular infection causes a pustular, ulcerative lesion. In the former instance, production of bloody and/or purulent sputum results. If systemic seeding occurs from these sites, abscesses in a variety of organs (or a diffuse maculopapular rash that may mimic smallpox) may arise. The pulmonary form of glanders occurs after inhalation of the bacterium or as a result of hematogenous spread from a focal infection. The incubation period in the respiratory tract is up to fourteen days. Clinical manifestations include fever, myalgias, headache, and pleuritic chest pain. The septicemic form of glanders can occur as a primary infection, or it may result from hematogenous spread from pulmonary or focal infections. Symptoms include flu-like features such as fever, myalgias, cervical adenopathy, and diarrhea, as well as cutaneous findings such as necrotizing, pustular lesions, generalized erythroderma, and jaundice. CNS findings of septicemic glanders include headache and photophobia. This form of the disease is distinctly uncommon but carries with it a high mortality rate with death occurring within 2 weeks of onset.
A bioterrorist attack with B. mallei would likely come in the form of an aerosol, and pulmonary forms would no doubt predominate. Further, signs and symptoms might occur faster with perhaps a higher likelihood of progression to the septicemic form. For the purposes of syndromic surveillance, glanders is best grouped with the flulike bioterrorism syndromes.
| Three acute forms: focal, pulmonary, and septic |
| Focal—nodular lesion with adenopathy; possible progression to pulmonary or septic forms |
| Pulmonary—myalgias, headache, and pleuritic chest pain |
| Septic—typical sepsis features as well as meningeal features. This form is associated with a high mortality. |
| Any of these forms can include a maculopapular rash secondary to bacterial seeding. |
Chronic Effects
Farcy, the chronic form of glanders, presents years or decades following the acute infection. Abscesses involving abdominal organs (e.g., liver and spleen), skeletal muscles, bone, or the skin may develop. These finding may be associated with lymphangitis and lymphadenopathy in the affected area. CNS involvement may occur with meningitis, encephalitis, or brain abscesses.
Laboratory Tests
Microscopy
Radiographic Findings
Differential Diagnosis
Because of the highly nonspecific nature of the symptoms caused by glanders, a broad differential must be assembled. The differential diagnosis must obviously be altered to the clinical presentation. Among others, tuberculosis must be considered with the pulmonary form, and smallpox must be considered if the dermatological features are present. A partial list is included in Table 20–6.
Diagnosis
The most specific diagnostic test available for glanders is complement fixation, and it is considered positive if the titer is greater than or equal to 1:20. However, this is not as sensitive as agglutination titers which have poor specificity. PCR would be needed to distinguish between glanders and melioidosis, the latter caused by the related microbe, Burkholderia pseudomallei.
Treatment
There is surprisingly little clinical experience treating glanders infection. The U.S. military’s recommended management for the focal form and pulmonary form are amoxicillin/clavulanate (20 mg/kg tid), tetracycline (40 mg/kg po tid) or trimethoprim-sulfamethoxazole (TMP 2 mg/kg, sulfa at 10 mg/kg bid). Regardless of which regimen is selected, the recommended course is 2 to 5 months. If the clinical picture is more severe, oral treatment should include two of the three standard regimens for 1 month followed by monotherapy for an additional 2 to 5 months. Treatment is prolonged 10 to 12 months further if pulmonary glanders is present or if abcesses have formed. The U.S. military’s recommended management for the septicemic form is 2 weeks of intravenous therapy with ceftazidime (40 mg/kg/day every 8 hours) plus TMP (2 mg/kg/day) and sulfa (10 mg/kg/day) every 6 hours. After 2 weeks, parenteral administration may be changed to oral administration and continued for an additional six months.
Vaccine
Infection Control
Glanders is not transmitted from person to person, and so standard infection control precautions are sufficient. If there is skin compromise—laceration or open sores—contact precautions are indicated. Adequate decontamination of surfaces can be achieved with standard bleach or disinfectants. Clothes and laundry that come into contact with cutaneous sites infected with glanders should also be cleaned.
Melioidosis (Burkholderia pseudomallei)
Background
Though closely related to Burkholderia mallei genetically and clinically, Burkholderia pseudomallei is distinctive in a number of important ways. Melioidosis, also known as Whitmore’s disease, is endemic to much of Southeast Asia and is not uncommon in other tropical regions. The disease takes multiple forms in humans including a septic form that is almost always fatal. A chronic form of melioidosis may activate years after the initial exposure, and is itself potentially life-threatening. Both the United States and the former Soviet Union attempted to weaponize B. pseudomallei, but the outcome of this research and development effort is unknown. It is generally agreed that as a biological weapon, B. pseudomallei will be used in aerosolized form.
Sources
B. pseudomallei bacteria are found in worldwide distribution in soil and water, particularly in rice paddies. Like with glanders, horses are a natural reservoir for B. pseudomallei, along with goats, monkeys, and rodents. The microbe’s presence in soil and water is thought to represent the primary route of exposure for humans. Australian outbreaks have been associated with potable water sources.
Properties
B. pseudomallei is a motile, flagellated facultative intracellular pathogen. It is resistant to dry heat and survives for long periods under arid conditions. The bacteria are saprophytic, surviving by attaching to organic debris in soil, streams, pools, stagnant water, rice paddies, and even vegetables. Land and marine animal reservoirs excrete and shed the bacteria, and this represents the primary means by which the bacterium is spread.
Epidemiology
Means of Transmission
Infection occurs as a result of either direct contact with contaminated objects or via material that enters the body through epidermal breaks, such as lacerations, scratches, or abrasions. The other common means are ingestion of contaminated water or inhalation of contaminated dust or particles. As a biological weapon, most experts feel that exposure would occur via an aerosolized form of the bacteria. Based on the natural history of sporadic melioidosis, the majority of those infected from a deliberate biological attack should not become ill. They would, however, be at a significant risk for relapse—particularly if immunosuppression or other co-morbid conditions exist or develop in the infected host.
Pathogenesis
The pathophysiology of melioidosis is poorly understood. As with Q fever, brucellosis, and glanders, it is an intracellular pathogen that thrives inside the lysosomes of macrophages. Similar to glanders, the bacteria resists phagocytic destruction, but unlike glanders, no exotoxins have yet been identified to explain this effect. Speculation that the virulence of melioidosis is related to the presence of a particular surface lipopolysaccharide has not been confirmed scientifically. Epitheliod granuloma formation is a common finding at infection sites. Bacteremic seeding often results in deep-tissue abscess formations.
Signs and Symptoms
Melioidosis is sometimes referred to as a “mimicker” because of its wide clinical manifestations. Like glanders, melioidosis occurs in three forms: focal, pulmonary, and septic. These may occur distinctly or in combination. Additionally, there is a chronic form that occurs following an incubation period that runs from a few days to a few years. The focal form usually results from direct contact between a contaminant and a break in the host’s epidermis. Nodular or pustular regional lymphadenopathy, myalgias, malaise, and fevers are often associated with dermal melioidosis. The lesion is painful, unlike cutaneous anthrax which is painless. The most common naturally occurring form of melioidosis is pulmonary. This results from inhalation of the microbe or hematogenous spread from a focal infection. Pneumonia with the formation of solitary or multiple lung abscesses are seen, usually with caseating necrosis. Involvement of the upper lobes of the lung is not uncommon, putting tuberculosis into the differential diagnosis. Cough is common, but is not invariably productive and if productive, it is rarely purulent. Myalgias, fevers, headaches, and weakness round out the clinical symptoms associated with pulmonary melioidosis. Septicemic melioidosis may occur as a primary infection, or again following pulmonary or focal infections. As with glanders, it is associated with a mortality rate greater than 90%. The clinical picture is that of septic shock, with hypotension and multiorgan system failure. Septicemic melioidosis is more likely in a host with significant co-morbidities, including diabetes, HIV, or chronic obstructive pulmonary disease (COPD). Pustular cutaneous lesions, myalgias, and deep tissue abscesses can occur anywhere throughout the body, including the central nervous system.
| Wide ranging clinical manifestations—fever, myalgias, arthralgias |
| Three forms—focal, pulmonary, and septic |
| Focal—painful nodular/pustular lesions, fever, myalgia |
| Pulmonary—pneumonia, possible pulmonary abscess in any part of lung, cough, fever |
| Septic—typical sepsis picture along with deep tissue abscesses |
Vulnerable Groups
Individuals with exposure to water in endemic areas of Southeast Asia and Northern Australia are more likely to develop melioidosis. Individuals with diabetes, liver failure, or renal disease, alcoholism, COPD, and other immunocompromised states are at increased risk for contracting the disease. These groups experience increased case fatality rates as well.
Chronic Effects
Chronic melioidosis occurs in infected persons who remained asymptomatic—and therefore untreated—during the acute period. It is a multisystem illness affecting about 3% of those infected with the bacterium. Any organ of the body—joints, viscera, muscle, lymph nodes, skin, brain, liver, lung, bones, and spleen—may be involved. Lymphadenopathy and lymphangitis may be seen proximal to the abscess site.
Laboratory Tests
Microscopy
B. pseudomallei is a non-spore-forming, gram-negative, bipolar (safety-pin), flagellate bacillus. Isolation of the bacteria in culture is key to making the diagnosis. Purulent sputum or discharge offers the highest yield, but negative cultures are common. Meat and sugar supplementation of the culture medium may stimulate growth of the bacterium.
Radiographic Findings
Differential Diagnosis
Diagnosis
Treatment
Antibiotic therapies for melioidosis follow the same regimes as for those of glanders. The U.S. military’s recommended management for the focal form and pulmonary form are amoxicillin/clavulanate (20 mg/kg/day every eight hours) or tetracycline (40 mg/kg/day in three divided oral doses) or TMP (2 mg/kg/day) with sulfa (10 mg/kg/day every 12 hours). Regardless of the regimen selected, the recommended course is two to five months. If the clinical picture is more grave, oral treatment should include two out of the three drugs for one month followed by monotherapy for an additional two to five months. An additional ten to twelve months is needed if, as with the pulmonic form, abscesses are present with drainage. The U.S. military’s recommended management for the septicemic form is two weeks of intravenous therapy with ceftazidime (40 mg/kg/day every 8 hours) plus TMP (2 mg/kg/day); and sulfa, (10 mg/kg/day every 6 hours). After 2 weeks, administration may be changed to oral forms and continued for an additional six months. In the septicemic form, empiric evidence supports the addition of granulocyte colony stimulating factor (GCSF).
Infection Control
Person-to-person transmission is possible through contaminated fluids. Contact precautions are therefore required. If sputum is culture positive, or while cultures are pending, respiratory isolation is suggested until confirmation of melioidosis, whereupon respiratory isolation is not necessary. Surface decontamination with standard cleaning agents is adequate. Medical equipment in contact with the patient should be autoclaved.
Of Note . . .
In the first 8 months of 2004, 79 people in Singapore became ill with melioidosis, leading to the death of 24. Typically, there are about 45 cases each year in the island nation. The increased numbers aroused suspicion among public health officials there, as did the unusually high mortality rate of nearly 30%, making it nearly twice as deadly as SARS. Although the increased number of infections and the increased case fatality rate were worrisome to local officials, this clustered outbreak prompted a government investigation to determine whether the infections were actually a result of deliberate exposures or bioterrorism. The Singapore government was particularly concerned because melioidosis is classified as a Category B agent. Results of the investigation showed no evidence of B. pseudomallei having been used as a biological weapon. The increased mortality was found to be a result of infection of people already bearing the burden of co-morbid conditions, such as diabetes mellitus, hypertension, or renal insufficiency. Although the increased mortality rate may thus be explained, the reasons for the increased incidence are still unclear.
Psittacosis (Chlamydia psittaci)
Background
Chlamydia psittaci is a member of the Chlamydia genus which is currently under taxonomical scrutiny because of its peculiar microbiological features. C. psittaci infection results in acute respiratory illness and may reactivate many years following acute infection. Seventy-five percent of all naturally occurring cases or outbreaks have been linked directly back to contact with or near birds carrying the bacteria. There are documented cases of C. psittaci infection having resulted from humans kissing birds and other cases from doing mouth-to-mouth resuscitation on birds. However, the other 25% appear to have had no exposure to birds. The largest outbreak in the Unites States occurred in 1930 when over 800 individuals became infected with psittacosis. As a biological weapon, C. psittaci has two potentials: the first as an aerosolization targetting human populations and the second as a deliberate agroterrorist attack on the poultry industry.
Sources
Classically, and most commonly, C. psittaci is found in bird droppings. Infection occurs from exposure to the droppings of healthy specimens serving as a reservoir. If there were widespread use of C. psittaci as a biological weapon, birds could sustain an epidemic by serving as natural reservoirs. The term psittacosis is somewhat of a misnomer as it implicates parrots as the primary source, whereas “ornithosis” would be a more inclusive and accurate term. To be sure, parrots tend to carry one of the more virulent forms of the disease, as do turkeys. Other animal reservoirs include livestock, for example, cattle and sheep; and domesticated animals, including cats and dogs.
Properties
C. psittaci is a gram-negative bacillus that functions as an obligate intracellular parasite. The bacteria survives unprotected for several days in bird excrement and can be spread as a wind-blown aerosol from dried feces. C. psittaci is a hardy organism, resistant to drying, and remaining viable for months under common environmental conditions.
Epidemiology
C. psittaci occurs in worldwide distribution. There are roughly 200 cases diagnosed annually in the United States. This number is probably an underestimate given the diagnostic difficulty of identifying a disease whose presentation is often subtle and mild. Birds function as the primary reservoir, but other animal reservoirs provide opportunity to cross species to infect humans. It has been reported that samplings of bird populations show a baseline infectivity rate of nearly 8%; this can rise 10-fold during outbreaks at commercial poultry settings.
Means of Transmission
Infection most commonly occurs as a result of inhalation of contaminated bird feces in the form of dust. Inhalation of contaminated respiratory or urinary products can lead to infection, as well. Cases of person-to-person transmission are unusual, but have been documented, often presenting with a more severe illness than those acquired from avian or animal sources.
Pathogenesis
The precise mechanism of C. psittaci is not understood, but the general actions are described. Chlamydia takes on two distinct morphologies: elementary and reticulate. The elementary form, or elementary body, is the infective form. The reticulate body is the proliferative form. Once in the airways, the elementary form of the bacterium adheres to the epithelial cells lining the respiratory tract and are endocytosed. As with the other obligate intracellular pathogens described earlier in this chapter, C. psittaci inhibits phagocytic mechanisms once inside the host cell. Within the endocytotic vesicle, the elementary body transforms into the reticulate body. This form of the microbe is responsible for bacterial reproduction, capable of producing hundreds of new bacteria inside the host cell. The bacteria cannot synthesize its own energy sources, so this replication consumes the host’s stores of energy. When bacterial replication reaches a maximum load, the cell ruptures and proximal cells are infected with the newly released bacteria. Cell lysis also results in hematogenous dissemination to the host’s reticuloendothelial system.
Signs and Symptoms
C. psittaci’s incubation period is between seven and fourteen days, but it can take as long as five weeks. Symptoms begin rapidly, gradually, or may remain subclinical and range from mild to severe, including death. Once again, the signs and symptoms are typically nonspecific, including fever, chills, sweats, myalgia, malaise, sore throat, headache, photophobia, and gastrointestinal distress. The textbook presentation of psittacosis is that of an atypical pneumonia—fever, nonproductive cough (often late appearing), malaise, dyspnea, and commonly a maculopapular rash. Physical examination may reveal rales, hepatomegaly, or splenomegaly.
Chronic Effects
Laboratory Tests
The leukocyte count is often within normal; however, an increase in band forms and neutrophilia is common. Liver function studies show transaminase elevations (commonly aspartate aminotransferase, or AST) and a concomitant drop in albumin levels. Serum chemistries may demonstrate elevation in blood urea nitrogen and creatinine levels and moderate hyponatremia.
Microscopy
Radiographic Findings
Chest radiographs in C. psittaci infection are usually normal. When abnormal, the CXR classically reveals lower lobe consolidation, often disproportionate to the clinical syndrome. That is, the patient may appear mildly ill, but have substantial lung involvement based on CXR findings. Less common radiographic findings include segmental consolidations or miliary patterns. As is common with other pneumonic processes, radiographic clearing often occurs weeks after clinical resolution.
Differential Diagnosis
The differential diagnosis for C. psittaci is vast. It is probably best included in the bioterror syndrome of flulike illnesses. Characteristically, it is a febrile respiratory illness and ranks among the short list of classic atypical pneumonias, such as Legionella and mycoplasma pneumonia. The differential also includes the panoply of bacterial, viral, and fungal pneumonias. Viral illnesses such as influenza and respiratory viruses, as well as rickettsial diseases and mycobacterial diseases (e.g., tuberculosis), are also included in the differential. The key to diagnosing psittacosis is careful social and environmental history taking. Close contact to animals—particularly birds—is a useful clue that can increase clinical suspicion for the disease. This questioning is even more essential if a possible bioterror attack is being investigated. A partial differential diagnosis for C. psittaci is included in the following table.
Diagnosis
Diagnosis of C. psittaci using complement fixation testing is a the gold standard. A fourfold rise in C. psittaci antibodies over a 2-week period confirms the diagnosis. Antibody testing does not distinguish among different chlamydial species, however. Definitive diagnosis of C. psittaci requires microimmunofluorescence of IgM titers greater than 1:16, or a fourfold increase in titers over a 2-week period.
Treatment
Tetracyclines are first-line agents in chlamydial infections. A 2-week treatment with doxycycline (100 mg orally every 12 hours) is the standard regimen. Critically ill patients should receive intravenous doxycycline (4.4 mg/kg every 12 hours). Typically, clinical improvement occurs in the first 48 hours after initiating antibiotic therapy.
Prophylaxis
There is no vaccine for C. psittaci, and postexposure prophylaxis is not recommended. Instead, exposed individuals should be placed under medical surveillance. Development of fever or other symptoms should lead to implementation of antibiotic treatment. Prior infection with C. psittaci does not confer lifetime immunity.
Infection Control
Typhus Fever (Rickettsia prowazekii)
Background
Infection with Rickettsia prowazekii leads to a clinical presentation often referred to as “typhus fever” or “epidemic typhus,” or the reactivation of a prior infection termed Brill–Zinsser disease. In its natural form, the bacteria is passed on in the feces of body lice (Pediculus humanus corporis) to individuals on whom the lice reside. Epidemics of typhus have been described through much of human history and occur whenever crowding and poor sanitary conditions exist. Natural outbreaks are more likely to occur in less industrialized nations, often as a result of the dislocation and social disruption incident to natural disasters or war. During WWI, tens of millions of typhus infections occurred in combatants with roughly 3 million deaths.
Sources
Typhus is a global infectious disease. It is an arthropod-borne disease, being carried in body lice who have themselves become infected by feeding on infected hosts. Hosts include humans and animals, such as flying squirrels.
After the acute illness resolves, infection with R. prowazekii may become subclinical as the microte remains hidden in undetermined sites in the body, and may reactivate at some later point, causing Brill-Zinsser disease. Patients with latent typhus infection may then serve as a resevoir of infection, capable of passing on, via the louse vector, to other individuals.
Properties
The mortality rate in untreated typhus is approximately 50%, whereas fatal cases are rare with treatment. Rickettsia are obligate intracellular bacteria. They survive for long periods in lice feces. The ID50—the dose required to cause infection in 50% of those exposed—is remarkably low. Just a few organisms are sufficient to cause disease. The hardiness of the microbe and its low ID50 make it a viable candidate for use as a biological weapon.
Epidemiology
Disease caused by R. prowazekii is found worldwide, although it is extremely uncommon in the United States. The last case in the United States was in 2001 in New Mexico, but no source could be identified. Outbreaks of typhus follow natural disasters such as floods and famines, and manmade disasters such as war.
Means of Transmission
Person-to-person transmission does not occur; rather body lice serve as the vector for the disease. Lice acquire the bacteria after biting an infected person during the first five days of symptoms. The bacteria take up residence and proliferate within the louse gastrointestinal tract and are then passed out in its abundant feces. The louse bites the human host causing a local inflammatory response with histamine release. The resulting pruritis elicits scratching which brings contaminated feces or contaminated louse body parts into the lesion effectively inoculating the host. Mucus membrane contact with contaminated lice feces also results in infection. It is speculated that inhalation of dust contaminated with louse feces can cause infection as well. Typhus epidemics, however, generally require crowded conditions, direct contact, or shared clothing; otherwise spread is difficult because infected lice tend to live only about 5 days before dying from the infection. An outbreak could also result from previously uninfected lice becoming infected by biting someone with a reactivation of a prior infection (Brill–Zinsser disease).
Pathogenesis
After inoculation from a louse bite, the bacteria are taken up into the capillaries where they then adhere to and invade the endothelial cells lining of vessels in which they are carried. Once inside, the bacteria proliferate until the bacterial load reaches maximum capacity and the cell bursts. Subsequent injury causes a local vasculitis and thrombogenesis with fibrin and platelet deposition at the rupture site. Thrombosis may continue from this point, with potential for thrombotic clots and damage to vital organs, the heart, or the kidneys.
Signs and Symptoms
After an incubation period of approximately 10 days, patients experience the sudden onset of signs and symptoms that last as long as 28 days if untreated. Findings include high fever, chills, weakness, cough, myalgias, abdominal pain, and headache. A rash beginning centrally and spreading centrifugally starts at about Day 4 with discrete macular lesions that evolve into petechial or necrotic lesions. These skin changes spare palmar and plantar surfaces. Severe CNS features can occur after 5 to 7 days of illness including mental status changes and meningitis. When present, gangrene occurs cutaneously or in the extremities depending on the size and location of thrombi formation.
| Abrupt onset of high fever, chills, weakness, cough, myalgias, abdominal pain, and headache |
| Discrete macular lesions that begin truncally and spread peripherally evolving into petechial or necrotic lesions |
| CNS features can occur with meningeal signs and symptoms and/or mental status changes |
| Thrombi formation occurs; signs and symptoms result depending on the size and location of the thrombi. Gangrene may be seen in peripheral or cutaneous thrombi formation |