Clinical Vignette
You are a pediatrician in an affluent suburban community. The mother of a 12-year-old boy brings him in for urgent evaluation. The youngster has been ill with fever, headache, and malaise for 4 days. His family has just returned from a two-week camping vacation in New Mexico. He appears wan and fatigued but is alert and oriented. Examination reveals fever and a slightly erythematous ear drum. You diagnose otitis media and initiate antibiotics. Two days later, his distraught mother calls saying that her son’s fever is worse, with relentless vomiting and severe abdominal pain. Suspecting appendicitis, the boy is referred to the ER. Surgery consultation is called for, and routine labs, including CBC, LFTs, and blood cultures are drawn. His WBC count is 25K with 60% bands, and he is taken to surgery. Intraoperatively, his appendix appears normal, but diffuse retroperitoneal and mesenteric lymphadenopathy is observed. He subsequently develops sepsis, ARDS, and DIC and is transferred to the ICU. In the ICU, he complains of severe headache and appears disoriented. His fingers are black, purpuric lesions are seen on his trunk and upper arms and Kernigs and Brudzinki’s signs are positive. That night, the lab calls stating that his blood cultures are growing gram-negative rods with a “safety pin” appearance. A rapid DFA sent to the State Health Department confirms Yersinia pestis.
Background
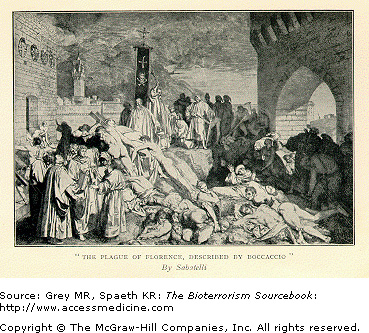
The first documented pandemic of Yersinia pestis occurred in 561 ad and is often called the Justinian Plague after the Holy Roman Emperor at the time. It began in Egypt and spread along trade routes killing upwards of 60% of the populations of Europe, North Africa, and southern and central Asia. The second great Yersinia outbreak, the so-called “Black Death,” occurred in 1346 and killed 25 million people, a third of the population of Europe (Fig. 15–1). Both pandemics were fueled by existing land and sea trade among countries in Europe, Asia, and Africa. The third pandemic began in China in 1855 and eventually spread worldwide. There continue to be intermittent outbreaks of plague throughout the world, although these outbreaks are isolated and better controlled primarily because of higher standards of living, improved sanitation and hygiene, and the availability of antibiotics.
Yersinia pestis is a nonmotile, non-spore-forming, gram-negative rod. Infection with Y. pestis tends to take one of three forms: bubonic, septicemic, and pneumonic. Historically the bubonic form of plague has been the most common. Classically, the disease is transmitted from infected rodents to humans by fleas residing on rats. As a biological weapon, pneumonic plague spread through aerosolization is expected to be the predominant form (Figs. 15–2 and 15–3).
Biblical Plague
Then the Lord said to Moses and Aaron, “Take for yourselves handfuls of soot from a kiln, and let Moses throw it toward the sky in the sight of Pharaoh. And it will become fine dust over all the land of Egypt, and will become boils breaking out with sores on man and beast through all the land of Egypt.” So they took soot from a kiln, and stood before Pharaoh; and Moses threw it toward the sky, and it became boils breaking out with sores on man and beast. And the magicians could not stand before Moses because of the boils, for the boils were on the magicians as well as on all the Egyptians.
Exodus 8–9
Yersinia as a Bioweapon
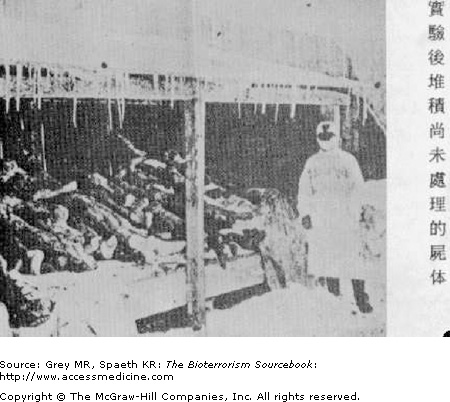
As Japan’s use of plague in China during WWII demonstrated, the effective use of plague as a bioweapon requires little sophistication and can have dramatic effects (Fig. 15–4). Most countries engaged in biological weapons research and development programs aim to use aerosolized Yersinia, eliminating the need for the rat host or flea vector and enhancing transmissibility and virulence. In 1970, the WHO estimated that 50 kg (110 lb) of Y. pestis sprayed over a city would infect 150,000 people and kill roughly 40,000. Both North Korea and Iraq are believed to have been engaged in weaponization of plague. The former Soviet Union’s Biopreparat conducted massive research on weaponized plague and large quantities were stockpiled. It is sobering to acknowledge that many scientists involved in this research, and the stores themselves, remain largely unaccounted for at present.
Distinguishing naturally occurring plague from a bioterrorist attack can be aided by epidemiologic clues, but it may not be readily apparent at the onset. Large numbers of cases occurring temporarily or spatially are highly suggestive of a biological attack. Crucial additional clues are the diagnosis of plague in areas with nonexistent, or very limited, historical experience, or in cities where no known zoonotic reservoir exists. Infection in patients with no discernible risk factors—such as exposure in research or occupational settings—or plague cases in absence of recent documentation of rodent dieoffs are useful clues as well.
The clinical form of plague might itself suggest either a naturally occurring zoonotic form or a deliberate attack. For example, naturally occurring epidemics of the pneumonic form of the plague are extremely rare and should raise suspicion of bioterrorism. Because the signs and symptoms of weaponized plague may be broad, primary care physicians must be cognizant of zoonotic and weaponized forms. Even with a plausible zoonotic source, any diagnosis of plague should raise the possibility of bioterrorism in today’s society.
In the past 50 years there have been fewer than 2,000 cases of plague in the United States. The clinical presentation of naturally occurring plague in the last 50 years has been dominated by the less deadly bubonic form (over 80% of all cases) with overall mortality at 14%. Another 13% of plague cases were septicemic with a mortality rate of 22%. Less than 5% of all U.S. plague cases presented in the pneumonic form, although the mortality rate is much higher (57%).
Populations at higher risk for naturally occurring plague are rural dwellers, hunters, campers, and American Indians who live in endemic areas of the Southwest. The presence of plague among prairie dogs and other domesticated animals represents a potential risk to rural dwellers, veterinarians, veterinary assistants, and others involved in animal control efforts, as well as pet store employees and pet owners.
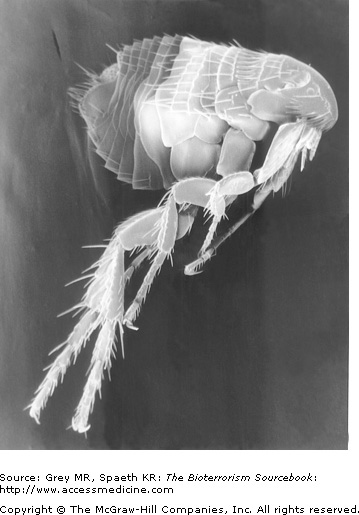
Naturally occurring plague transmits to humans from bites of infected fleas that typically derived their infection through having bitten infected rodents (Fig. 15–5). Generally, such transmission results in the bubonic form of the plague. A small percentage of those bitten, however, will develop a septicemic form referred to as primary septicemic plague. It is important to note that neither of these two forms are transmitted from one human to another. Those infected with a pneumonic form, in contrast, can spread the disease through aerosolized droplets. In a biological attack, the most likely means of transmission would be through aerosolization of plague bacilli. Consequently, the clinical scenario would be the opposite of that seen with naturally occurring plague. That is, the pneumonic form would dominate and mortality would be far higher than with naturally-occurring plague. The success of such an attack would still be influenced by a number of variables, including climatic conditions, or the amount and type of Y. pestis strain used. It is possible for a biological attack to come in the form of deliberate infection of a natural animal reservoir in a major city. However, experts believe this is an inefficient means of initiating a plague epidemic.
When a flea ingests Y. pestis from an infected source, such as a rat, the bacterium undergoes replication in the flea’s esophagus. The esophagus becomes blocked, ultimately, causing regurgitation of the bacterium when it is feeding on hosts, thus spreading the disease. In zoonotic transmission, bites from plague-carrying fleas introduce upward of a thousand organisms into the dermis (Fig. 15–6). Once present in the skin, Y. pestis makes its way through the lymphatics to regional lymph nodes where rapid proliferation results in lymph node necrosis. Bacteremic spread from regional lymph nodes and septicemia and shock may follow unless timely treatment is initiated.