Clinical Vignette
A large local employer for whom you do preplacement examinations calls to say that an employee in the mailroom saw an envelope with a suspicious white powder coming from its seams. Following the CDC guidelines, the company protocol for suspicious mail is followed: the envelope is left undisturbed and the room is vacated and locked. Although identification of the substance is pending, the company president has many questions, including whether his employees must be given a vaccine or antibiotics and wants your advice. What should you say?
Background
Since antiquity, anthrax has affected livestock and humans. The name anthrax derives from the Greek word for “coal” because of the black skin lesions it can cause. One of the first records of anthrax is in the Old Testament’s Book of Exodus as the fifth and possibly sixth of the ten plagues (Fig. 14–1). Virgil wrote verses on the disease in 25 bc describing the toll it took on livestock and the health risks to humans from exposure to infected meats and skins. The modern history of anthrax begins in the late nineteenth century when Louis Pasteur identified the bacterium responsible for anthrax, an observation that lent early credibility to the germ theory of disease. Anthrax was used as a weapon in World War I as a means to cause economic havoc through the loss of livestock.
Anthrax (Bacillus anthracis) is a gram-positive, nonmotile spore-forming bacterium that infects both humans and animals, particularly livestock. Anthrax is found in a worldwide distribution. The spores, most commonly found in soil, are hardy and may survive for decades. In humans, the disease presents in three forms: inhalational, cutaneous, and gastrointestinal. Mortality is greatest for the inhalational form. If diagnosed early, anthrax is a highly treatable disease. Even weaponized anthrax, such as that used in the attacks through the U.S. Postal Service, is treatable with conventional antibiotics.
Epidemiology
The incidence of anthrax has dropped significantly over the years in technologically advanced countries, in large part because of vaccination of high-risk groups—those regularly exposed to hides, wools, and other raw livestock products. Prior to the anthrax attacks in 2001, American experience with anthrax was limited. The only modern experience with the inhalational form was in 1979 with the accidental release of anthrax spores from the Soviet bioweapons facility in Sverdlosk, Russia, in which 72 died. The last case of inhalational anthrax in the United States occurred in 1972.
Worldwide the disease is more common, with best estimates ranging between 20,000 and 100,000 cases of anthrax annually, and almost all occurring in developing nations. Most current public health policies regarding anthrax, such as vaccination strategies and medical management human experience are based on limited information and are ever evolving. The mortality rates for inhalational anthrax are greater than 80%; however, this figure was arrived at prior to modern antibiotics and the advent of critical care resources. The post-September 11 inhalational anthrax patients had a mortality rate of 40% (Table 14–1).
| In 1970, the WHO estimated that 50 kg of anthrax spores upwind of a major city would result in 125,000 cases of anthrax and 95,000 deaths. |
| In 1979, an accidental release of weaponized anthrax from Sverdlosk, USSR, resulted in 77 cases of anthrax and 72 deaths. |
| Has potential for mass casualties as well as more limited covert attack (e.g., U.S. Post Office). |
| After the September 11 anthrax attacks, 13 cases were diagnosed, 5 died, and over 10,000 individuals were placed on post-exposure prophylaxis |
Occupational Risk Groups
Vulnerable Groups
Immunocompromised individuals (e.g., HIVS/AIDS, patients undergoing cancer treatment), diabetics, children, the elderly, and individuals with preexisting respiratory conditions should be considered at greater risk (Fig. 14–2). Pregnant women may be treated safely, but dosing recommendations vary.
Means of Transmission
Infection occurs from direct contact with active bacteria or indirectly from contact with spores that then germinate. The spores may be in animal hair, meat, hides, or other products. The weaponized form of anthrax is constituted as spores less than five microns in size and may be maintained as powder or aerosolized. Prior to the U.S. Postal Service attacks, the LD50 (see Glossary) for anthrax was thought to be between 2,500 and 55,000 inhaled spores. Weaponized anthrax is far more potent with inhalation of as few as several hundred spores sufficient to cause disease. Estimates from the U.S. Postal Service attacks suggest that the LD50 as well may have been an order or two below naturally occurring anthrax (Fig. 14–3, Table 14–1).
Pathogenesis
B. anthracis derives its virulence from the presence of a capsule and from three secreted proteins: protective antigen, edema factor, and lethal factor. Protective antigen facilitates the binding of the bacteria to host cell membranes and subsequent transport intracellularly of the other two toxins. Edema toxin acts to inhibit neutrophils as well as causes edema by disrupting water homeostasis. Lethal toxin causes activation and dysregulation of cytokines such as interleukin-1 and tumor necrosis factor (TNF).
Microscopy
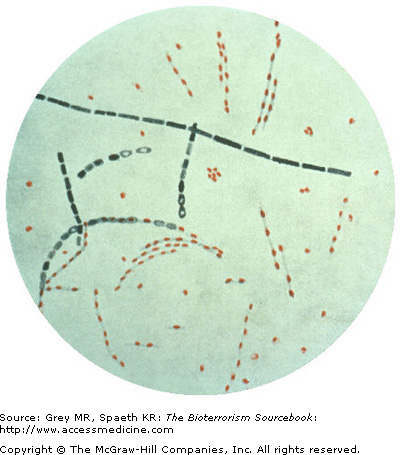
Key identifying features include gram-positive rods measuring approximately 1 to 1.5 × 3 to 5 microns singly or in chains, with a “bamboo” or boxcar appearance (Fig. 14–4). General features include nonmotile, nonhemolytic, and encapsulated bacterium easily demonstrated with India ink culture. Anthrax spores are 1 to 1.5 microns in size and oval in shape. They grow readily on ordinary laboratory media at body temperature.
Though few laboratories specialize in diagnosis of bioweapon agents per se, diagnosis can generally be made based on the features of B. anthracis as a gram-positive, spore-forming bacillus. However, PCR, ELISA, and other assays are now available to confirm the presence of anthrax. Anthrax specimens may be handled in a BSL-2 laboratory, that is, a standard clinical laboratory that practices “universal” precautions and has facilities for minimizing aerosols. Spore handling requires a BSL-3 laboratory.
Clinical Presentation
Inhalational Anthrax
Pathophysiology
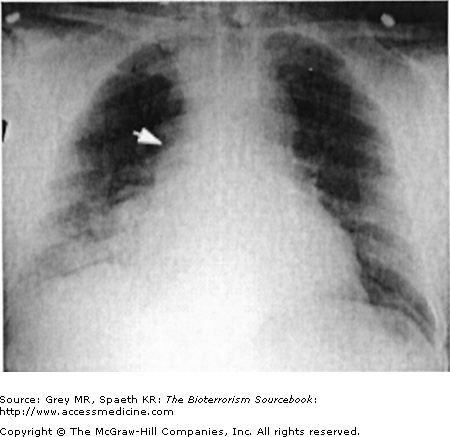
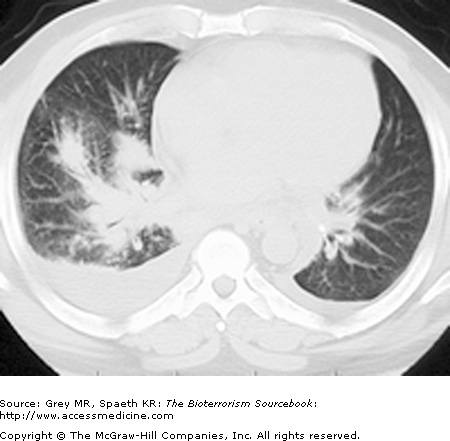
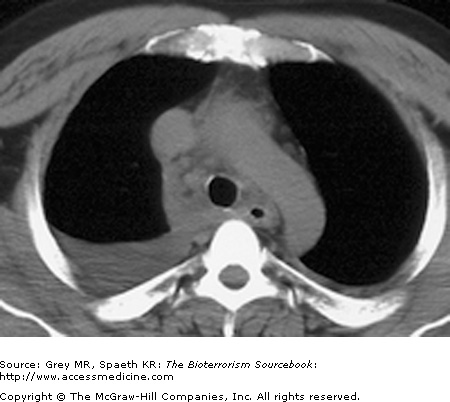
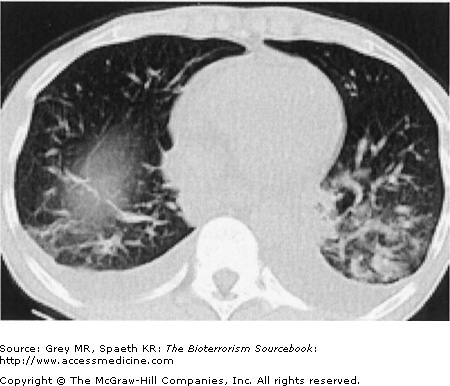
Classically, spores located in the soil or in contaminated animal products are kicked up into ambient air and inhaled directly into the respiratory tract. As a weapon, anthrax may be distributed in powdered or aerosolized forms. Anthrax spores are the ideal size for inhalation deep into the lung. Once inhaled, they easily reach the alveolar spaces where most are phagocytized and destroyed by alveolar macrophages. Surviving spores are taken up by the lymphatics and arrive at the mediastinal lymph nodes where the spores undergo germination into bacillus form. Germination may take up to six weeks longer, complicating both clinical diagnosis and, if the result of a bioterror release, the public health response. Once germination begins, both local and systemic symptoms of inhalational anthrax appear rapidly, resulting from the release of bacterial toxins. The toxins cause necrotic, edematous, and hemorrhagic thoracic lymphadenitis and a subsequent systemic inflammatory response. It is the toxin level that is associated with mortality, not the bacteria itself. This is significant because a negative blood culture does not necessarily mean the patient has reached a point of improvement. Pathologically, the bronchopneumonic processes noted are distinctive in inhalational anthrax, with a hemorrhagic mediastinitis that is virtually pathognomonic. Pleural effusions are common and often require aggressive drainage (Figs. 14–5, 14–6, 14–7, and 14–8).
Signs and Symptoms
Inhalational anthrax may present in a biphasic pattern (early and late) or as a continuously progressive disease. The weaponized form used in the post-September 11 anthrax attacks followed this biphasic pattern. On the whole, the victims’ signs and symptoms were consistent with those seen in the classic zoonotic form. Though it was a small sample, there were two notable differences between classic anthrax and what was seen in the attacks: the inhalational rather than cutaneous form predominated, and infectivity occurred despite inhalation of spore levels below previously known pathogenic levels.
The early stages of inhalational anthrax lasts hours to days. The prominent early clinical findings in ten of eleven of the post-September 11 anthrax victims were myalgia and fever. The constellation of signs and symptoms includes fever, myalgia, nonproductive cough, nausea, vomiting, diaphoresis, dyspnea, myalgia, chest pain, headache, and tachycardia. Other associated symptoms include chills and abdominal pain.
Two to three days following the onset of Stage I, late signs and symptoms evolve. Stage II inhalational anthrax is presaged by respiratory paralysis, diaphoresis, cyanosis, acute fever splices, hypotension, respiratory alkalosis and terminal acidosis, massive lymphadenopathy, hemorrhagic meningitis (often with concurrent meningismus), delirium, and obtundation. This stage progresses rapidly to shock, hypothermia, and death within 24 to 36 hours. The transition from Stage I to Stage II can occur suddenly, as a continuum, or even after a short period of improvement.
Laboratory Tests
Traditional teaching was that sputum and blood cultures are an insensitive means of diagnosing anthrax in the inhalational form, particularly sputum cultures, because there is often little in the way of a pneumonic process taking place. However, in the Swerdlovsk and U.S. Postal Service outbreak, pretreatment blood cultures were positive in all instances. Once bacteremia or systemic infection is established, sputum stains and cultures may be of value. Standard laboratory testing in the early stages of anthrax is largely unhelpful. For example, there is no or minimal leukocytosis. Identification of classic “boxcar”-shaped gram-positive bacilli from sputum or blood culture in the proper setting would make anthrax a primary diagnostic possibility. Buffy coat stains may yield higher sensitivity. ELISA testing for toxin antibodies can be confirmatory. PCR is also available.
Radiographic Findings
Inhalational anthrax is characterized by a series of radiographic and tomographic changes that can aid greatly in diagnosis. Radiographs provide critical diagnostic information many hours or even days before blood or sputum cultures can turn positive. These changes, when presenting late, indicate a poor prognosis. Computed tomography (CT) taken of the post-September 11 anthrax patients also showed characteristic abnormalities that have substantial diagnostic value. Initial CTs in virtually all of the post-September 11 inhalational anthrax cases were markedly abnormal and had an unusual combination of findings of enlarged hyperattenuating mediastinal and hilar lymph nodes, diffuse mediastinal fat edema, peribronchial thickening, and pleural effusions. Given the rarity of inhalational anthrax in the modern era, this terrorist attack provided valuable information for the presentation of anthrax in the era of CT scans. The findings corroborate the classic chest radiograph findings. CT scans are now considered a standard diagnostic tool for inhalational anthrax. These key radiographic findings are summarized in Table 14–2 (see also Figs. 14–5, 14–6, 14–7, and 14–8).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree