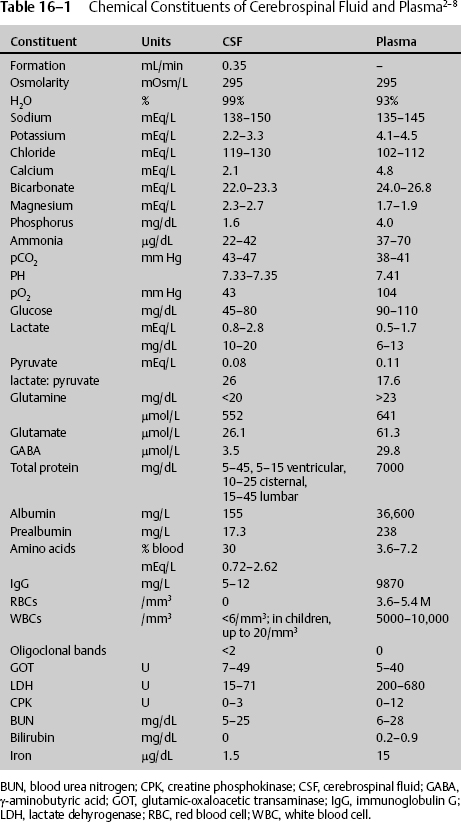
16 Lynn M. Serrano, John D. Cantando, and Dan Miulli Cerebrospinal fluid (CSF) is found within the four ventricles of the brain, the subarachnoid space, and the central canal of the spinal cord. It is also called liquor cerebrospinalis.1 CSF circulates a variety of chemicals and nutrients necessary for normal brain function and metabolism; it also acts as a shock absorber, cushioning the brain from both day-to-day activity and traumatic events. Grossly, CSF should be a colorless, odorless, serous fluid. There is an estimated 70 to 160 cc of fluid in the central nervous system at any time (~50% intracranial, 50% spinal). Certain pathological conditions will change both the chemical and gross appearance of CSF. In the majority of cases, it is simple to ascertain whether fluid is CSF or not. However, at times, especially when contaminated with other fluids, it is necessary to analyze the fluid for its constituents to determine if an unknown fluid is CSF, contains CSF, or is another body fluid.2–8 Table 16–1 compares the composition of CSF with plasma. To determine if a fluid is CSF, the following tests can be done: Cerebrospinal fluid constituents are affected by secretion and absorption rates of CSF, hormones, and chemicals. The secretion rates and effects of hormones and chemicals on CSF vary from the vascular to the ventricular side of the choroid plexus.13–15 These are described in Table 16–2. CSF from blood plasma is actively transported by the choroid plexus (80%), or invaginations of the pia mater, into the ventricles, with the remaining 10 to 20% produced by ventricular ependymal cells, brain parenchyma, and indirect cellular fluid shifts. The approximate CSF secretion is 450 cc per day, which corresponds to a rate of 0.3 (0.35–0.37) mL/minute. The flow of CSF is in constant movement in a continuous pattern. Starting from the choroid plexus in the lateral ventricles, CSF continues through the foramen of Monro into the third ventricle and passes into the cerebral aqueduct prior to the fourth ventricle. From the fourth ventricle, fluid escapes into the cisterns and subarachnoid space via the foramen of Luschka and the foramen of Magendie. Some enters the central canal of the spinal cord, although most spinal fluid then circulates through the subarachnoid space and is reabsorbed in the venous system via the arachnoid villi. To keep the total spinal fluid in circulation throughout the ventricular system and subarachnoid space at ~150 cc, the absorption into the venous system is relatively constant at 450 cc/day, matching the daily production. There should be at least 3 to 5 mm Hg pressure of CSF for absorption to take place. Pathological states can alter the production, secretion, and/or circulatory flow of CSF.
Cerebrospinal Fluid Dynamics and Pathology
 Cerebrospinal Fluid Identification
Cerebrospinal Fluid Identification
 Chemical Regulators of Cerebrospinal Fluid
Chemical Regulators of Cerebrospinal Fluid
 Flow Pattern of Cerebrospinal Fluid
Flow Pattern of Cerebrospinal Fluid
| Vascular side of choroid plexus |
Nonadrenergic sympathetic innervation (near CP epithelial cells and blood vessels) decreases CSF flow by 30%. Cholinergic input primarily near the third ventricle stimulates CSF production up to 100%. Endothelin binding sites are found in CP of lateral and third ventricles. Endothelin decreases blood flow and subsequently CSF production. Antidiuretic hormone (ADH) regulates norepinephrine, dopamine, and endorphin release within the ventricle. ADH has been shown to indirectly decrease plasma Na+. |
| Ventricular side of choroid plexus |
5-hydroxytryptamine (5HT) The CP contains 10 times the amount of 5HT receptors relative to other areas of the brain. It is released from the supraependymal nerve fibers into the CSF and interacts with the CP-5HT receptors. 5HT reduces the rate of CSF secretion. Melatonin binding sites are located in the fourth ventricle and stimulate CSF secretion. Carbonic anhydrase High concentrations within the CP increase CSF production by facilitating Na+ transport. L-dopa is the most abundant monoamine in the CSF. The CP has D1 receptors but lacks direct dopaminergic innervation. Dopamine effects on the CP are via the CSF, similar to 5HT. Norepinephrine is secreted by noradrenergic periventricular neurons in contact with the ventricles and decreases CSF production. It follows circadian variations similar to systemic circulation. Arginine vasopressin (AVP) is released by vasopressinergic neurons into the CSF, which stimulates CSF production. AVP in the CSF follows circadian variations, whereas plasma levels do not. The CP has V1 receptors for AVP. AVP has been ii shown to indirectly lower plasma Na+. Arial natriuretic peptide (ANP) reduces CSF production. It is elevated in hydrocephalus cases. Evidence supports ANP involvement in the regulation of water and electrolyte passage across the blood–brain barrier. ANP has a direct negative effect on CSF production as substantiated by increased levels of ANP circulating within the CSF in hydrocephalic patients (both normal and high pressure). Systemically, ANP stimulates renal inhibition of Na+ and water absorption, leading to hyponatremia. Within the brain, ANP reduces the net flux of Na+ from the circulation by inhibiting the Na+/K+/Cl− cotransport system that is known to decrease CSF production.8,9 |
CP, choroid plexus; CSF, cerebrospinal fluid.
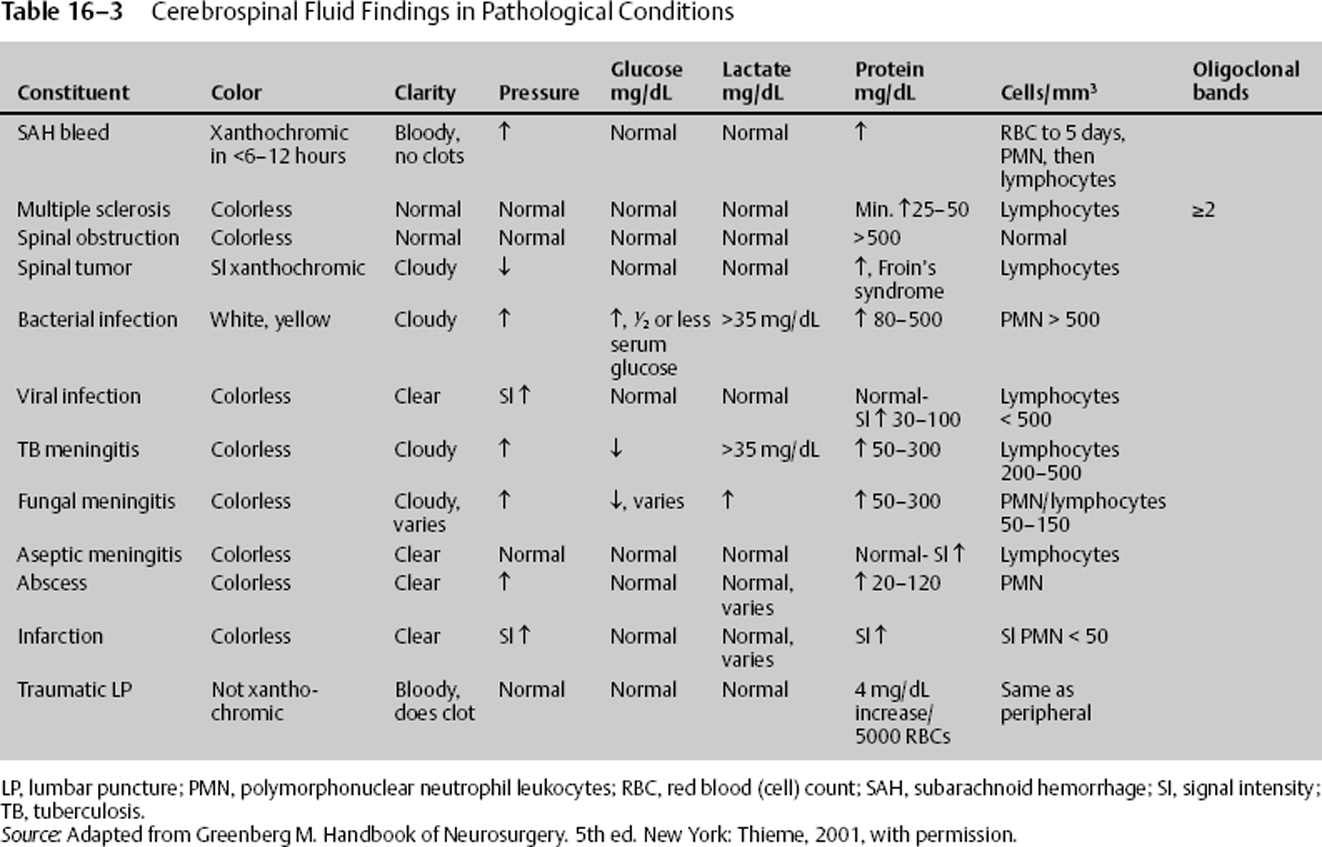
 Pathology Involving Cerebrospinal Fluid
Pathology Involving Cerebrospinal Fluid
Table 16–3 notes changes in the gross appearance and the chemical composition of CSF due to certain diseases states.
Disorders of Volume and Pressure
Normal pressure hydrocephalus (NPH) is associated with a classic triad of symptoms: dementia, gait disturbance, and urinary incontinence. The etiology is usually idiopathic but can be secondary to other intracranial pathology, such as Alzheimer’s disease, carcinomatosis, infectious meningitis, and subarachnoid hemorrhage.16 Diagnosis is primarily clinical, with documented normal pressure via lumbar puncture and a full workup of other causes of dementia. Some clinicians augment clinical symptoms by performing a quantitative lumbar puncture. Usually a cognitive assessment, such as a neuropsychological test, precedes the lumbar puncture. The lumbar puncture measures the opening pressure, allows 20 to 40 cc of fluid to drain off, then measures the closing pressure. If the brain is normally compliant when the change in volume divided by the change in pressure is ~0.62, the closing pressure may be reduced by 0.45 cm of CSF pressure for every 1 cc of CSF removed. Thus removing 30 cc of fluid should reduce closing pressure by ~13 cm CSF (10 mm Hg). This is the adult normal pressure-volume index (PVI; 25–30 mL change in volume causes a 10-fold change in pressure in mm Hg). No change in CSF pressure may indicate poor cerebral compliance, low PVI, and increased intracranial pressure; a large lowering in CSF pressure may indicate low intracranial pressure, herniation, or complete block of CSF pathways. Following the lumbar puncture, there should be a repeat cognitive assessment, such as a neuropsychological test, to determine if the quantitative lumbar puncture resulted in clinical improvement. Some clinicians do not rely on this test, whereas others will not shunt questionable cases of NPH without a positive improvement in cognitive assessment after a quantitative lumbar puncture. Treatment of NPH is shunting, either ventricular or, at rare times, lumbar-peritoneal.
Communicating and noncommunicating hydrocephalus symptoms include nausea, vomiting, gait disturbance, frontal headache (frequently worse in the morning), paresis of upward gaze, disorders of sodium, and papilledema. Temporizing measures for relief may include a ventricular catheter and/or diuretics (acetazolamide or furosemide). Permanent treatment should be directed at the offending pathology; however, frequently, a CSF-diverting procedure, such as a shunt or third ventriculostomy, is required.16–20
Obstructive (noncommunicative) hydrocephalus is blockage of the normal flow of CSF, causing dilatation of the ventricles proximal to the obstruction.
Triventricular hydrocephalus is specifically a stenosis occurring at the sylvian aqueduct, yielding dilatation of both lateral ventricles and the third ventricle. Common etiologies include edema, mass effect, mass lesion, and congenital abnormality.
Communicating (nonobstructive) hydrocephalus is a disruption of the equilibrium of secretion and absorption of CSF, yielding increased volume of CSF. It is most commonly caused by malabsorption of the CSF by the arachnoid granulations. Common etiologies include infection, hemorrhage, trauma, and noninfectious meningitis.
| CSF pressure > 20 cm H2O |
| Normal CSF composition |
| Symptoms of elevated intracranial pressure without focal deficit |
| Normal imaging studies (occasionally slit ventricles may be seen) |
CSF, cerebrospinal fluid.
Pseudotumor cerebri (idiopathic benign intracranial hypertension) symptoms may include nausea, vomiting, headache, retro-orbital pain, visual changes, including blindness (may be permanent) associated with increased intracranial pressure, possible optic atrophy, and, when progressive, papilledema (Table 16–4).
Treatment includes medical management with diuretics (acetazolamide or furosemide); if refractory, surgical management is warranted, with serial lumbar punctures, shunting, or optic nerve decompression.21
Leptomeningeal or Arachnoid Cysts
Leptomeningeal cysts are congenital fluid collections between two layers of the arachnoid. They are not related to post-traumatic leptomeningeal cysts due to growing skull fractures. There are two types of arachnoid cysts classified by histological findings: (1) simple, in which the lining of the cyst consists of cells capable of secreting CSF (this is the most common type of middle fossa arachnoid cyst); and (2) complex, in which the lining of the cyst is multicellular, often containing neuroglia and ependyma.
Classic presentation is in early childhood, when there is a sudden onset associated with hemorrhagic conversion or cyst rupture. Symptoms and presentation vary according to location and mass effect; asymptomatic lesions are usually identified incidentally (Table 16–5).
Diagnosis is via computed tomography (CT) scan or magnetic resonance imaging (MRI). Most cysts are static, however; repeat imaging can be used to rule out cystic changes or enlargement. Treatment is indicated only when the patient is symptomatic and other etiologies have been ruled out. Most common treatments are shunting using a low-pressure valve, which has a low rate of reoccurrence, marsupialization, or a combination of both (Table 16–6).4,5
| Location | Signs and symptoms |
| Middle fossa 50% of cysts in adults, 30% of cysts in children | Asymptomatic; unilateral headaches, nausea/vomiting, seizures, mild hemiparesis, present at younger age, male:female ratio 3:1, more in left hemisphere, hemorrhage |
| Suprasellar 9% of cysts | Increased ICP, hydrocephalus, craniomegaly, developmental delay, precocious puberty, bobbing head, visual loss |
ICP, intracranial pressure.
Infectious and Noninfectious Irritants Causing Meningitis
Post-traumatic meningitis is usually limited to head trauma with an associated skull fracture. Organisms are most commonly gram-positive cocci and gram-negative bacilli. Treatment should be directed at the offending agent (Table 16–7).2,3,6,7,9,10
Trauma-Related Cerebrospinal Fluid Abnormalities
Infectious
See Chapter 24, Table 24-2, cerebrospinal fluid analysis.
Cerebrospinal Leak
CSF leaks are associated with basal skull fractures and anterior fossa fractures resulting in otorrhea and/or rhinorrhea. Diagnosis is made by clinical exam; however, contrasted CT scans and radionuclide cisternograms can help identify the source of the leak. Analysis is required (see Table 16–1) to confirm the fluid as CSF. Treatment consists of general measures to lowering intracranial pressure, raising the head of the bed, acetazolamide to decrease CSF production, lumbar drain insertion, and/or surgical repair. Surgical repair is indicated in refractory and recurrent CSF leaks.
Pneumocephalus is evidence of air intracranially. Air can be intraparenchymal, intraventricular, subdural, or epidural. It is associated with a skull defect or injury to the tegmen tympani (congenital/traumatic/related to pressure changes, e.g., deep-sea diving). The skull defect can be congenital, postprocedural, or post-traumatic. Pneumocephalus must be closely monitored with frequent CT scans to confirm resolution. Prophylactic antibiotic use is controversial. A tension pneumocephalus is the result of expanding trapped gas and can be associated with gas producing bacterial infection, room temperature air expanding due to increased body temperature after sealing the access, and the continued use of nitrous oxide anesthesia gas after the closure of the dura.
| Cyst type | Description | Treatment |
| Type I | Communicates with subarachnoid space | No treatment, follow-up imaging every 6 months for 18 months |
| Type II | Large, quadrangular, mass effect; delayed uptake with cisternogram contrast | Surgery if symptoms severe; surgery either cystoperitoneal shunting or cyst marsupial fenestration |
| Type III | Large, round, mass effect; no communication with subarachnoid space; bone expansion of middle fossa | Surgery if symptoms severe; either cystoperitoneal shunting or cyst marsupial fenestration |
| Patient population | Organism | Suggested antibiotics |
| Neonates (<1 month) | Group B/D Streptococcus Enterobacteriaceae Listeria | Ampicillin Gentamycin (alt. third-generation cephalosporin) |
| Newborns (1–3 months) | Pneumococci Meningococci Haemophilus influenzae | Ampicillin Third-generation cephalosporin +/− dexamethasone |
| Children (3 months–7 years) | Pneumococci Meningococci H. influenzae | Third-generation cephalosporin (alt. ampicillin) |
| Older children (>7 years) and Adults | Streptococcus pneumoniae Neisseria Meningococci | Third-generation cephalosporin Ampicillin (in combination with resistance, add rifampin +/−vancomycin |
| Alcoholics, immunocomprised, and elderly | Pneumococci Enterobacteriaceae Pseudomonas Listeria | Vancomycin Third-generation cephalosporin |
| Postprocedural | Staphylococcus aureus Enterobacteriaceae Pseudomonas Pneumococci | Vancomycin Ceftazidime +/− gentamycin |
Traumatic Lumbar Puncture
Traumatic lumbar puncture can occur during a procedure to obtain CSF; local trauma or disruption of nearby vascular structures can produce a traumatic tap. The CSF analysis will still be accurate in most pathologies; however, its appearance can complicate the diagnosis of subarachnoid hemorrhage. When CT scan is negative for subarachnoid hemorrhage, yet the patient history and physical exam are highly suspicious, a lumbar puncture can be used to limit the differential diagnosis. Table 16–8 itemizes the characteristics of a traumatic tap.
| Decline in number of RBCs with succeeding tubes |
| WBCs proportional to blood RBCs. |
| Blood will clot. |
| No xanthochromia if first attempt within 2–12 hours, unless protein > 150 mg/dL or RBCs > 1.5 M/mm3 or high lipid levels |
| Xanthochromia appears in the CSF within 2 hours in limited cases, 6 hours 70% of time, and 12 hours 90% of time. |
| Protein consistent with plasma or increased above-normal CSF levels by 1 mg/1000 RBCs. |
CSF, cerebrospinal fluid; RBC, red blood cell; WBC, white blood cell.
< div class='tao-gold-member'>