Central Venous Catheterization
Scott A. Celinski
Michael G. Seneff
Placement of central venous catheters (CVC) remains one of the most commonly performed procedures in the intensive care unit (ICU). Since the publication of the previous edition of this textbook, a significant milestone in CVC management was achieved with the report of a sustained zero incidence of catheter-related infection (CRI) in a clinical setting of a complicated medical-surgical ICU [1]. That experience was duplicated by several other medical-surgical ICUs across the state of Michigan participating in the Keystone Project. Of significant note, this zero incidence of CRI was primarily achieved not through new catheter technology but rather by adherence to strict catheter insertion and maintenance protocols. The implication is that any ICU can duplicate these experiences, independent of budget or specialty, using appropriate management and empowerment of bedside caregivers. Inexplicably, many ICUs still have not incorporated these protocols into standardized daily practices. In this chapter, we review the techniques and complications of the various routes available for central venous catheterization and present a strategy for catheter management that incorporates all of the recent advances.
Indications and Site Selection
Technical advances and a better understanding of anatomy have made insertion of central venous catheters easier and safer, but there is still an underappreciation of the inherent risks. Like any medical procedure, central venous catheterization has specific indications and should be reserved for the patient who has potential to benefit from it. After determining that CVC is necessary, physicians often proceed with catheterization at the site they are most experienced with, which might not be the most appropriate route in that particular patient. Table 2-1 lists general priorities in site selection for different indications of CVC; the final choice of site in a particular patient should vary based on individual institutional and operator experiences.
Volume resuscitation alone is not an indication for CVC. A 2.5-inch, 16-gauge catheter used to cannulate a peripheral vein can infuse twice the amount of fluid as an 8-inch, 16-gauge central venous catheter [2]. However, peripheral vein cannulation can be impossible in the hypovolemic, shocked individual. In this instance, the subclavian vein (SV) is the most reliable central site because it remains patent due to its fibrous attachments to the clavicle. Depending on the clinical situation, the femoral vein (FV) is a reasonable alternative, but the risk of deep venous thrombosis always needs to be considered [3,4,5 and 6].
Central venous access is often required for the infusion of irritant medications (concentrated potassium chloride) or vasoactive agents, certain diagnostic or therapeutic radiologic procedures, and in any patient for whom peripheral access is not possible.
Long-term total parenteral nutrition is best administered through SV catheters, which should be surgically implanted if appropriate. The internal jugular vein (IJV) is the preferred site for acute hemodialysis, and the SV should be avoided because of the relatively high incidence of subclavian stenosis following temporary dialysis, which then limits options for an AV fistula should long-term dialysis become necessary [7,8]. The FV is also suitable for acute short-term hemodialysis or plasmapheresis in nonambulatory patients [9].
Emergency transvenous pacemakers and flow-directed pulmonary artery catheters are best inserted through the right IJV because of the direct path to the right ventricle. This route is associated with the fewest catheter tip malpositions. For patients with coagulopathy, the external jugular vein (EJV) is an acceptable alternative, but we rarely find it necessary. The SV is an alternative second choice for pulmonary artery catheterization, even in many patients with coagulopathy [10], but the left SV is preferred to the right SV because of the less acute turns required to reach the heart. The reader is referred to Chapter 4 for additional information on the insertion and care of pulmonary artery catheters.
Preoperative CVC is desirable in a wide variety of clinical situations. One specific indication for preoperative right ventricular catheterization is the patient undergoing a posterior craniotomy or cervical laminectomy in the sitting position. These patients are at risk for air embolism, and the catheter can be used to aspirate air from the right ventricle [11]. Neurosurgery is the only common indication for an antecubital approach, as IJV catheters are in the operative field and theoretically can obstruct blood return from the cranial vault and increase intracranial pressure. Subclavian catheters are an excellent alternative for preoperative neurosurgical patients if pneumothorax is ruled out prior to induction of general anesthesia.
Venous access during cardiopulmonary resuscitation warrants special comment. Peripheral vein cannulation in circulatory arrest may prove impossible, and circulation times of drugs administered peripherally are prolonged when compared to central injection [12]. Drugs injected through femoral catheters also have a prolonged circulation time unless the catheter tip is advanced beyond the diaphragm, although the clinical significance of this is debated. Effective drug administration is an extremely important element of successful cardiopulmonary resuscitation; venous access should be established as quickly as possible, either peripherally or centrally if qualified personnel
are present. Prolonged attempts at arm vein cannulation are not warranted, and under these circumstances the FV is a good alternative because, despite the potential of longer drug circulation times, cardiopulmonary resuscitation (CPR) is interrupted the least with its placement. If circulation is not restored after administration of appropriate drugs and defibrillation, central access should be obtained by the most experienced operator available with a minimum interruption of CPR [13].
are present. Prolonged attempts at arm vein cannulation are not warranted, and under these circumstances the FV is a good alternative because, despite the potential of longer drug circulation times, cardiopulmonary resuscitation (CPR) is interrupted the least with its placement. If circulation is not restored after administration of appropriate drugs and defibrillation, central access should be obtained by the most experienced operator available with a minimum interruption of CPR [13].
TABLE 2-1. Indications for Central Venous Catheterization | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The placement of central venous catheters is now also specifically indicated for patients with severe sepsis, septic shock, or acute respiratory distress syndrome (ARDS), in order to monitor central venous oxygen saturation (ScvO2 [14] and central venous pressure (CVP) [15]. Rivers et al. [14] showed a 16% absolute reduction of in-hospital mortality with early goal directed therapy for patients with severe sepsis, which included keeping the ScvO2 greater than 70%. Early goal-directed therapy was subsequently shown to be achievable in “real world” settings [16]. For these patients, the relationship between superior vena caval and inferior vena caval oxygen saturations has not been elucidated; therefore, at this time we recommend superior vena caval catheterization through the SV, IJV, or EJV for this patient population. Likewise, the ARDS network recently reported that CVP monitoring utilizing a CVC is as effective as a pulmonary artery catheter in managing patients with acute lung injury and ARDS [15,17]. Since many of these patients are on high levels of positive end expiratory pressure (PEEP) and at high risk for complications from pneumothorax, the IJV or EJV represents the safest approach, especially in the absence of an experienced operator.
General Considerations and Complications
General considerations for CVC independent of the site of insertion are catheter tip location, vascular erosions, catheter-associated thrombosis, air and catheter embolism, and coagulopathy, which are discussed below. Catheter-associated infection is discussed separately.
Catheter Tip Location
Catheter tip location is a very important consideration in CVC. The ideal location for the catheter tip is the distal innominate or proximal superior vena cava (SVC), 3 to 5 cm proximal to the caval-atrial junction. Positioning of the catheter tip within the right atrium or right ventricle should be avoided. Cardiac tamponade secondary to catheter tip perforation of the cardiac wall is uncommon, but two thirds of patients suffering this complication die [18]. Perforation likely results from vessel wall damage from infused solutions combined with catheter tip migration that occurs from the motion of the beating heart as well as patient arm and neck movements. Migration of catheter tips can be impressive: 5 to 10 cm with antecubital catheters and 1 to 5 cm with IJV or SV catheters [19,20]. Other complications
from intracardiac catheter tip position include provocation of arrhythmias from mechanical irritation and infusion of caustic medications or unwarmed blood [21].
from intracardiac catheter tip position include provocation of arrhythmias from mechanical irritation and infusion of caustic medications or unwarmed blood [21].
Correct placement of the catheter tip is relatively simple, beginning with an appreciation of anatomy. The caval-atrial junction is approximately 16 to 18 cm from right-sided skin punctures and 19 to 21 cm from left-sided insertions and is relatively independent of patient gender and body habitus [22,23]. Insertion of a standard triple-lumen catheter to its full 20 cm frequently places the tip within the heart, especially following right-sided insertions. A chest radiograph should be obtained following every initial central venous catheter insertion to ascertain catheter tip location and to detect complications. The right tracheobronchial angle is the most reliable landmark on plain film chest radiograph for the upper margin of the SVC and is always at least 2.9 cm above the caval-atrial junction. The catheter tip should lie about 1 cm below this landmark, and above the right upper cardiac silhouette to ensure placement outside of the pericardium [24].
Vascular Erosions
Large-vessel perforations secondary to central venous catheters are uncommon and often not immediately recognized. Vessel perforation typically occurs 1 to 7 days after catheter insertion. Patients usually present with sudden onset of dyspnea and often with new pleural effusions on chest radiograph [25]. Catheter stiffness, position of the tip within the vessel, and the site of insertion are important factors causing vessel perforation. The relative importance of these variables is unknown. Repeated irritation of the vessel wall by a stiff catheter tip or infusion of hyperosmolar solutions may be the initiating event. Vascular erosions are more common with left IJV and EJV catheters, because for anatomical reasons the catheter tip is more likely to be positioned laterally under tension against the SVC wall [26]. Positioning of the catheter tip within the vein parallel to the vessel wall must be confirmed on chest radiograph. Free aspiration of blood from one of the catheter ports is not always sufficient to rule out a vascular perforation.
Air and Catheter Embolism
Significant air and catheter embolism are rare and preventable complications of CVC. Catheter embolism can occur at the time of insertion when a catheter-through- or over-needle technique is used and the operator withdraws the catheter without simultaneously retracting the needle. It more commonly occurs with antecubital or femoral catheters after insertion, because they are prone to breakage when the agitated patient vigorously bends an arm or leg. Prevention, recognition, and management of catheter embolism are covered in detail elsewhere [27].
Air embolism is of greater clinical importance, often goes undiagnosed, and may prove fatal [28,29]. This complication is totally preventable with compulsive attention to proper catheter insertion and maintenance. Factors resulting in air embolism during insertion are well known, and methods to increase venous pressure, such as use of the Trendelenburg position, should not be forgotten. In modern ICUs, catheter disconnect or passage of air through a patent tract after catheter removal are more common causes of catheter-associated air embolism. An air embolus should be suspected in any patient with an indwelling or recently discontinued CVC who develops sudden unexplained hypoxemia or cardiovascular collapse, often after being moved out of bed or to a stretcher. A characteristic mill wheel sound may be auscultated over the precordium. Treatment involves placing the patient in the left lateral decubitus position and using the catheter to aspirate air from the right ventricle. Hyperbaric oxygen therapy to reduce bubble size has a controversial role in treatment [28]. The best treatment is prevention, and prevention can be most effectively achieved through comprehensive nursing and physician-in-training educational modules and proper supervision of inexperienced operators [29]. Discontinuation of catheters should always be done with the patient supine.
Coagulopathy
Central venous access in the patient with a bleeding diathesis is problematic. The SV and IJV routes have increased risks in the presence of coagulopathy, but it is not known at what degree of abnormality the risk becomes unacceptable. A coagulopathy is generally defined as an international normalization ratio (INR) greater than 1.5 or platelet count less than 50,000. Thrombocytopenia is probably a greater risk than prolonged coagulation times. Although it is clear that safe venipuncture is possible with greater degrees of coagulopathy [10,30], even with the subclavian approach, the literature is also fraught with case reports of serious hemorrhagic complications. In patients with severe coagulopathy, the EJV is an alternative for central venous access, especially pulmonary artery catheterization, while the FV offers a safe alternative for general-purpose venous access. In appropriate patients, peripherally inserted central venous catheter (PICC) is useful. If these sites cannot be used, the IJV is the best alternative.
Thrombosis
Catheter-related thrombosis is very common but usually of little clinical significance. The spectrum of thrombotic complications includes a fibrin sleeve surrounding the catheter from its point of entry into the vein distal to the tip, to mural thrombus, a clot that forms on the wall of the vein secondary to mechanical or chemical irritation, or occlusive thrombus, which blocks flow and may result in collateral formation. All of these lesions are usually clinically silent; therefore, studies that do not use venography or color flow Doppler imaging to confirm the diagnosis underestimate its incidence. Using venography, fibrin sleeve formation can be documented in a majority of catheters, mural thrombi in 10% to 30%, and occlusive thrombi in 0% to 10% [31,32, 33,34,35, 36 and 37]. In contrast, clinical symptoms of thrombosis occur in only 0% to 3% of patients [6,32]. The incidence of thrombosis probably increases with duration of catheterization but does not appear reliably related to the site of insertion [6,31,32, 33,34,35, 36 and 37]. However, femoral vein catheter-associated thrombosis in the lower extremity is almost certainly more clinically important than upper extremity thrombosis caused by IJ and SV catheters [3,4 and 5]. The presence of catheter-associated thrombosis is also associated with a higher incidence of infection [36,37].
Catheter design and composition impact the frequency of thrombotic complications. The ideal catheter material is nonthrombogenic and relatively stiff at room temperature to facilitate percutaneous insertion, yet soft and pliable at body
temperature to minimize intravascular mechanical trauma. Not all studies are consistent, but polyurethane, especially when coated with hydromer, appears to be the best material available for bedside catheter insertions [32, 38, 39]. Silastic catheters have low thrombogenicity but must be surgically implanted, and pressure monitoring may not be possible. Heparin bonding of catheters decreases thrombogenicity and also appears to decrease the rate of catheter related infections [40]. Low dose heparin infused through the catheter or administered subcutaneously and very-low-dose warfarin therapy also decrease the incidence of venogram-proven and clinically apparent thrombosis [41].
temperature to minimize intravascular mechanical trauma. Not all studies are consistent, but polyurethane, especially when coated with hydromer, appears to be the best material available for bedside catheter insertions [32, 38, 39]. Silastic catheters have low thrombogenicity but must be surgically implanted, and pressure monitoring may not be possible. Heparin bonding of catheters decreases thrombogenicity and also appears to decrease the rate of catheter related infections [40]. Low dose heparin infused through the catheter or administered subcutaneously and very-low-dose warfarin therapy also decrease the incidence of venogram-proven and clinically apparent thrombosis [41].
Routes of Central Venous Cannulation
Antecubital Approach
The antecubital veins are used in the ICU for CVC with PICC and midline catheters. Use of PICCs in critically ill adults is limited by lack of surface anatomy in obese and edematous patients, lack of technological versatility (i.e., limited pressure monitoring [42], small lumens, and no triple-lumen capability), and increased time and decreased predictability of bedside insertion. PICCs are potentially useful in highly selected ICU patients undergoing neurosurgery, with coagulopathy, or in the rehabilitative phase of critical illness for whom general purpose central venous access is required for parenteral nutrition or long-term medication access (see Table 2-1) [43, 44]. The technique of percutaneous insertion of these catheters using the basilic, cephalic, or brachial vein is described below.
Anatomy
The basilic vein is formed at the ulnar aspect of the dorsal venous network of the hand (Fig. 2-1). It may be found in the medial part of the antecubital fossa, where it is usually joined by the median basilic vein. It then ascends in the groove between the biceps brachii and pronator teres on the medial aspect of the arm to perforate the deep fascia distal to the midportion of the arm, where it joins the brachial vein to become the axillary vein. The basilic vein is preferred for CVC because it is almost always of substantial size and the anatomy is predictable; since the axillary vein is a direct continuation of it, the basilic vein provides an unimpeded path to the central venous circulation [45, 46].
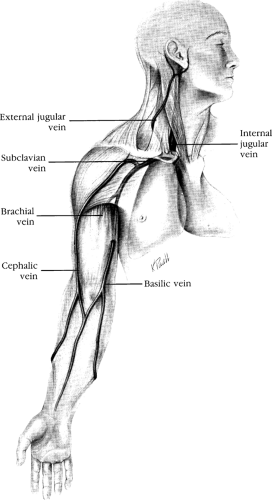 FIGURE 2-1. Venous anatomy of the upper extremity. The internal jugular, external jugular, and subclavian veins are also shown. |
The cephalic vein begins in the radial aspect of the dorsal venous network of the hand and ascends around the radial border of the forearm (see Fig. 2-1). In the lateral aspect of the antecubital fossa, it forms an anastomosis with the median basilic vein and then ascends the lateral part of the arm in the groove along the lateral border of the biceps brachii. It pierces the clavipectoral fascia in the deltopectoral triangle and empties into the proximal part of the axillary vein caudal to the clavicle. The variability of the cephalic vein anatomy renders it less suitable than the basilic vein for CVC. It joins the axillary vein at nearly a right angle, which can be difficult for a catheter to traverse. Instead of passing beneath the clavicle, the cephalic vein may pass through the clavicle, compressing the vein and making catheter passage impossible. Furthermore, in a significant percentage of cases, the cephalic does not empty into the axillary vein but divides into smaller branches or a venous plexus, which empties into the ipsilateral EJV. The cephalic vein may also simply terminate or become attenuated just proximal to the antecubital fossa [45, 46].
Technique of Cannulation
Several kits are available for antecubital CVC. The PICC and midline catheters are made of silicone or polyurethane and, depending on catheter stiffness and size, are usually placed through an introducer. The method described below is for a PICC catheter inserted through a tear-away introducer.
The right basilic vein should be selected for the initial attempt at CVC because of the above anatomical considerations and clinical studies that confirm a higher success rate with the basilic than the cephalic vein [47]. The success rates from either arm are comparable, though the catheter must traverse a greater distance from the left. With the patient’s arm at his or her side, the antecubital fossa is prepared and draped, adhering to strict
aseptic technique. A tourniquet is placed proximally and if an appropriate vein is not part of the surface anatomy, we use a portable ultrasound device to identify the basilic or its main branches. After local anesthesia, venipuncture is performed with the thin-wall entry needle proximal to the antecubital crease to avoid catheter breakage and embolism. When free backflow of venous blood is confirmed, the tourniquet is released and the guidewire carefully threaded into the vein for a distance of 15 to 20 cm. Leaving the guidewire in place, the thin-wall needle is withdrawn and the puncture site enlarged with a scalpel blade. The sheath-introducer assembly is then threaded over the guidewire with a twisting motion, and the guidewire is removed. Next, leaving the sheath in place, the dilator is removed, and the introducer is now ready for PICC insertion. The length of insertion is estimated by measuring the distance along the predicted vein path from the venipuncture site to the manubriosternal junction, using the measuring tape provided in the kit. The PICC is supplied with an inner obturator that provides stiffness for insertion, which must be inserted into the PICC after it has been flushed and before insertion. After measurement, the PICC is trimmed to the desired length, and the obturator is inserted into the PICC and advanced until the tips are equal. The PICC/obturator is then inserted through the introducer to the appropriate distance, the introducer peeled away, and the obturator removed. The PICC is then secured in place and a chest radiograph is obtained to determine tip position.
aseptic technique. A tourniquet is placed proximally and if an appropriate vein is not part of the surface anatomy, we use a portable ultrasound device to identify the basilic or its main branches. After local anesthesia, venipuncture is performed with the thin-wall entry needle proximal to the antecubital crease to avoid catheter breakage and embolism. When free backflow of venous blood is confirmed, the tourniquet is released and the guidewire carefully threaded into the vein for a distance of 15 to 20 cm. Leaving the guidewire in place, the thin-wall needle is withdrawn and the puncture site enlarged with a scalpel blade. The sheath-introducer assembly is then threaded over the guidewire with a twisting motion, and the guidewire is removed. Next, leaving the sheath in place, the dilator is removed, and the introducer is now ready for PICC insertion. The length of insertion is estimated by measuring the distance along the predicted vein path from the venipuncture site to the manubriosternal junction, using the measuring tape provided in the kit. The PICC is supplied with an inner obturator that provides stiffness for insertion, which must be inserted into the PICC after it has been flushed and before insertion. After measurement, the PICC is trimmed to the desired length, and the obturator is inserted into the PICC and advanced until the tips are equal. The PICC/obturator is then inserted through the introducer to the appropriate distance, the introducer peeled away, and the obturator removed. The PICC is then secured in place and a chest radiograph is obtained to determine tip position.
If resistance to advancing the PICC is met, options are limited. Techniques such as abducting the arm are of limited value. If a catheter-through or over-needle device has been used, the catheter must never be withdrawn without simultaneously retracting the needle to avoid catheter shearing and embolism. If the catheter cannot be advanced easily, another site should be chosen.
Success Rate and Complications
Using the above technique the PICC has a 75% to 95% successful placement rate, and success is increased with operator experience, identification of a large vein, or the use of fluoroscopy [43, 44, 48]. Overall, PICCs appear to be at least as safe as CVCs, but frequent occlusions and impaired flow are a significant limitation. Important complications include sterile phlebitis, thrombosis (especially of the SV and IJV), infection, limb edema, and pericardial tamponade. Phlebitis may be more common with antecubital central venous catheters, probably due to less blood flow in these veins as well as the proximity of the venipuncture site to the skin [49, 50]. The risk of pericardial tamponade may also be increased if the catheter tip is inserted too deep because of greater catheter tip migration occurring with arm movements [51]. Complications are minimized by strict adherence to recommended techniques for catheter placement and care.
Internal-External Jugular Approach
Pediatricians used the IJV for venous access long before Hermosura et al. [52] described the technique and advocated its use in adults in 1966. The first large series on IJV catheterization appeared in 1969, when English et al. [53] reported on 500 percutaneous IJV catheterizations. Reports confirming this route’s efficiency and low complication rate followed, and it has remained a popular site for central venous access.
Anatomy
The IJV emerges from the base of the skull through the jugular foramen and enters the carotid sheath dorsally with the internal carotid artery (ICA) (see Fig. 2-1). It then courses posterolaterally to the artery and runs beneath the sternocleidomastoid (SCM) muscle. The vein lies medial to the anterior portion of the SCM muscle superiorly, then runs beneath the triangle formed by the two heads of the muscle in its medial portion before entering the SV near the medial border of the anterior scalene muscle at the sternal border of the clavicle. The junction of the right IJV (which averages 2 to 3 cm in diameter) with the right SV forming the innominate vein follows a straight path to the SVC. As a result, malpositions and looping of a catheter inserted through the right IJV are unusual. In contrast, a catheter passed through the left IJV must negotiate a sharp turn at the left jugulosubclavian junction, which results in a greater percentage of catheter malpositions [54]. This sharp turn may also produce tension and torque at the catheter tip, resulting in a higher incidence of vessel erosion [25, 26].
Knowledge of the structures neighboring the IJV is essential as they may be compromised by a misdirected needle. The ICA runs medial to the IJV but, rarely, may lie directly posterior or anterior. Behind the ICA, just outside the sheath, lie the stellate ganglion and the cervical sympathetic trunk. The dome of the pleura, which is higher on the left, lies caudal to the junction of the IJV and SV. Posteriorly, at the root of the neck, course the phrenic and vagus nerves [45, 46]. The thoracic duct lies behind the left IJV and enters the superior margin of the SV near the jugulosubclavian junction. The right lymphatic duct has the same anatomical relationship but is much smaller, and chylous effusions typically occur only with left-sided IJV cannulations.
Techniques of Cannulation
Internal jugular venipuncture may be accomplished by a variety of methods; all methods use the same landmarks but differ in the site of venipuncture or orientation of the needle. Defalque [55] grouped the methods into three general approaches: anterior, central, and posterior (Fig. 2-2). We prefer the central approach for the initial attempt, but the method chosen varies with the institution and the operator’s experience. All approaches require identical equipment, and the operator may choose from many different catheters and prepackaged kits.
Standard triple-lumen catheter kits include the equivalent of a 7-French (Fr) triple-lumen catheter with 15 (recommended), 20, or 30 cm of usable length, a 0.032-inch diameter guidewire with straight and J tip, an 18-gauge thin-wall needle, an 18-gauge catheter-over-needle, a 7-Fr vessel dilator, a 22-gauge “finder”; needle, and appropriate syringes and suture material. Preparation of the guidewire and catheter prior to insertion is important; all lumina should be flushed with saline and the cap to the distal lumen removed. The patient is placed in a 15-degree Trendelenburg position to distend the vein and minimize the risk of air embolism, with the head turned gently to the contralateral side. The surface anatomy is identified, especially the angle of the mandible, the two heads of the SCM, the clavicle, the EJV, and the trachea (see Fig. 2-2). The neck is then prepared with chlorhexidine and fully draped, with the operator wearing a hat, mask, sterile gown, and gloves. It is recommended that all
catheter preparation be performed prior to draping the patient, as they often develop a sense of claustrophobia with draping.
catheter preparation be performed prior to draping the patient, as they often develop a sense of claustrophobia with draping.
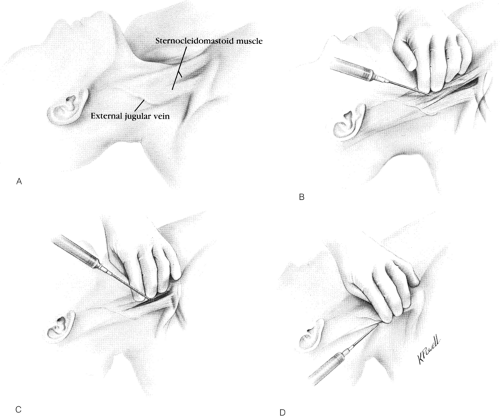 FIGURE 2-2. Surface anatomy and various approaches to cannulation of the internal jugular vein. A: Surface anatomy. B: Anterior approach. C: Central approach. D: Posterior approach. |
For the central approach [55], skin puncture is at the apex of the triangle formed by the two muscle bellies of the SCM and the clavicle. The ICA pulsation is usually felt 1 to 2 cm medial to this point, beneath or just medial to the sternal head of the SCM. The skin at the apex of the triangle is infiltrated with 1% lidocaine using the smallest needle available. Use of a small-bore finder needle to locate the IJV should prevent unintentional ICA puncture and unnecessary probing with a larger-bore needle. To avoid collapsing the IJV, the operator should maintain minimal to no pressure on the ICA with the left hand and insert the finder needle with the right hand at the apex of the triangle at a 45-degree angle with the frontal plane, directed at the ipsilateral nipple. The needle is advanced steadily with constant negative pressure in the syringe, and venipuncture occurs within 1 to 5 cm. If venipuncture does not occur on the initial forward thrust, negative pressure should be maintained and the needle slowly withdrawn, as often, the needle will compress the vein on the forward thrust, penetrating the back wall without blood return. Once the needle is pulled back past the posterior wall of the vessel, it achieves free flow of blood from the vessel. If the first attempt is unsuccessful, the operator should reassess patient position, landmarks, and techniques to ensure that he or she is not doing anything to decrease IJV lumen size (see below). Subsequent attempts may be directed slightly laterally or medially to the initial thrust, as long as the plane of the ICA is not violated. If venipuncture does not occur after three to five attempts, further attempts are unlikely to be successful and only increase complications [56, 57, and 58].
When venipuncture has occurred with the finder needle, the operator can either withdraw the finder needle and introduce the large-bore needle in the identical plane or leave the finder needle in place and introduce the larger needle directly above it. Leaving the finder needle in place has been shown to facilitate successful puncture with the introducer needle [59]. Many kits provide both an 18-gauge thin-wall needle through which a guidewire can be directly introduced and a 16-gauge catheter-over-needle device. With the latter apparatus, the catheter is threaded over the needle into the vein, the needle withdrawn,
and the guidewire inserted through the catheter. Both techniques are effective; the choice is strictly a matter of operator preference. Regardless of which large-bore needle is used, once venipuncture has occurred, the syringe is removed after ensuring that the backflow of blood is not pulsatile and the hub is then occluded with a finger to prevent air embolism or excessive bleeding. The guidewire, with the J-tip oriented appropriately, is then inserted and should pass freely up to 20 cm, at which point the thin-wall needle or catheter is withdrawn. The tendency to insert the guidewire deeper than 15 to 20 cm should be avoided, as it is the most common cause of ventricular arrhythmias during insertion and also poses a risk for cardiac perforation. Occasionally, the guidewire does not pass easily beyond the tip of the thin-wall needle. The guidewire should then be withdrawn, the syringe attached, and free backflow of blood reestablished and maintained while the syringe and needle are brought to a more parallel plane with the vein. The guidewire should then pass easily. If resistance is still encountered, rotation of the guidewire during insertion often allows passage, but extensive manipulation and force only lead to complications.
and the guidewire inserted through the catheter. Both techniques are effective; the choice is strictly a matter of operator preference. Regardless of which large-bore needle is used, once venipuncture has occurred, the syringe is removed after ensuring that the backflow of blood is not pulsatile and the hub is then occluded with a finger to prevent air embolism or excessive bleeding. The guidewire, with the J-tip oriented appropriately, is then inserted and should pass freely up to 20 cm, at which point the thin-wall needle or catheter is withdrawn. The tendency to insert the guidewire deeper than 15 to 20 cm should be avoided, as it is the most common cause of ventricular arrhythmias during insertion and also poses a risk for cardiac perforation. Occasionally, the guidewire does not pass easily beyond the tip of the thin-wall needle. The guidewire should then be withdrawn, the syringe attached, and free backflow of blood reestablished and maintained while the syringe and needle are brought to a more parallel plane with the vein. The guidewire should then pass easily. If resistance is still encountered, rotation of the guidewire during insertion often allows passage, but extensive manipulation and force only lead to complications.
With the guidewire in place, a scalpel is used to make two 90-degree stab incisions at the skin entry site to facilitate passage of the 7-Fr vessel dilator. The dilator is inserted down the wire to a depth that ensures that the soft tissues are sufficiently dilated to accept the catheter while maintaining control and sterility of the guidewire. Except in obese or edematous patients where the distance from the skin to the vessel may be large, the dilator should not be inserted to the hub in order to avoid vessel damage. The dilator is then withdrawn and pressure used at the puncture site to control oozing and prevent air embolism down the needle tract. The triple-lumen catheter is then inserted over the guidewire, ensuring that the operator has control of the guidewire, either proximal or distal to the catheter, at all times to avoid intravascular loss of the wire. The catheter is then advanced 15 to 17 cm (17 to 19 cm for left IJV) into the vein, the guidewire withdrawn, and the distal lumen capped. The catheter is sutured securely to limit tip migration and bandaged in a standard manner. A chest radiograph should be obtained to detect complications and tip location.
Alternative Approaches
The anterior and posterior approaches are identical in technique, differing only in venipuncture site and plane of insertion. For the anterior approach (see Fig. 2-2) [55, 60, 61], the important landmark is the midpoint of the sternal head of the SCM, approximately 5 cm from both the angle of the mandible and the sternum. At this point, the carotid artery can be palpated 1 cm inside the lateral border of the sternal head. The index and middle fingers of the left hand gently palpate the artery, and the needle is introduced 0.5 to 1 cm lateral to the pulsation. The needle should form a 45-degree angle with the frontal plane and be directed caudally parallel to the carotid artery toward the ipsilateral nipple. Venipuncture occurs within 2 to 4 cm, sometimes only while the needle is slowly withdrawn. If the initial attempt is unsuccessful, the next attempt should be at a 5-degree lateral angle, followed by a cautious attempt more medially, never crossing the plane of the carotid artery.
The posterior approach (see Fig. 2-2) [55, 62, 63, and 64] uses the EJV as a surface landmark. The needle is introduced 1 cm dorsally to the point where the EJV crosses the posterior border of the SCM or 5 cm cephalad from the clavicle along the clavicular head of the SCM. The needle is directed caudally and ventrally toward the suprasternal notch at an angle of 45 degrees to the sagittal plane, with a 15-degree upward angulation. Venipuncture occurs within 5 to 7 cm. If this attempt is unsuccessful, the needle should be aimed slightly more cephalad on the next attempt.
Success Rates
Internal jugular vein catheterization is associated with a high rate of successful catheter placement regardless of the approach used. Elective procedures are successful more than 90% of the time, generally within the first three attempts, and catheter malposition is rare [54, 55, 57, 60]. Operator experience does not appear to be as important a factor in altering the success rate of venipuncture as it is in increasing the number of complications [57, 65]. Emergent IJV catheterization is less successful and is not the preferred technique during airway emergencies or other situations that may make it difficult to identify landmarks in the neck.
Ultrasound studies have been useful in delineating factors that improve the efficiency of IJV cannulation. The ability to perform IJV venipuncture is directly proportional to its cross-sectional lumen area (CSLA); thus, these factors increase or decrease the vein’s caliber impact on the success rate [66, 67]. Factors that decrease the CSLA include hypovolemia, carotid artery palpation, and excessive tension on a finder needle. Predictably, the Valsalva maneuver and Trendelenburg position increase CSLA, as does high-level PEEP. There is also a progressive increase in CSLA as the IJV nears the SV. Overrotation of the neck may place the vein beneath the SCM muscle belly [66].
Often, cannulation is successful on the first attempt by optimizing CLSA through attention to the above measures. If the IJV is still not punctured after one or two attempts, it is usually because of anatomical variation, not because of the absence of jugular flow [67, 68]. In this situation, we use a portable ultrasound device to locate the IJV [69]. Whatever technique is employed, prolonged attempts at catheterization after optimization of IJV CSLA are only likely to increase complications.
Complications
The incidence and types of complications are similar regardless of the approach. Operator inexperience appears to increase the number of complications, but to an undefined extent, and probably does not have as great an impact as it does on the incidence of pneumothorax in subclavian venipuncture [55, 70].
The overall incidence of complications in IJV catheterization is 0.1% to 4.2% [53, 55, 62]. Important complications include ICA puncture, pneumothorax, vessel erosion, thrombosis, and infection. By far the most common complication is ICA puncture, which constitutes 80% to 90% of all complications. In the absence of a bleeding diathesis, arterial punctures are usually benign and are managed conservatively without sequelae by applying local pressure for 10 minutes. Even in the absence of clotting abnormalities, a sizable hematoma may form, frequently preventing further catheterization attempts or, rarely, exerting pressure on vital neck structures [71, 72]. Unrecognized arterial puncture can lead to catheterization of the ICA with a large-bore catheter or introducer and can have disastrous consequences,
especially if heparin is subsequently administered [73]. Management of carotid cannulation with a large-bore catheter, such as a 7-Fr introducer, is controversial. Options include pulling the catheter and applying pressure, percutaneous closure devices, internal stent grafting, or surgical repair [74, 75]. Some experts advise administration of anticoagulants to prevent thromboembolic complications, while others advise the opposite. Our approach is to remove small-bore catheters and avoid heparinization if possible, as hemorrhage appears to be a greater risk than thromboembolism [73]. For larger bore catheters and complicated cases, we involve interventional radiology and vascular surgery before removal and individualize the management based on the circumstances.
especially if heparin is subsequently administered [73]. Management of carotid cannulation with a large-bore catheter, such as a 7-Fr introducer, is controversial. Options include pulling the catheter and applying pressure, percutaneous closure devices, internal stent grafting, or surgical repair [74, 75]. Some experts advise administration of anticoagulants to prevent thromboembolic complications, while others advise the opposite. Our approach is to remove small-bore catheters and avoid heparinization if possible, as hemorrhage appears to be a greater risk than thromboembolism [73]. For larger bore catheters and complicated cases, we involve interventional radiology and vascular surgery before removal and individualize the management based on the circumstances.
Coagulopathy is a relative contraindication to IJV catheterization, but extensive experience suggests that it is generally safe [56]. In patients with clinical bleeding abnormalities, it is prudent to first proceed with EJV or FV catheterization, but if the IJV is considered most appropriate, a finder needle should always be used in an attempt to avoid ICA puncture with a larger needle, and ultrasound localization of the vessel is recommended.
Pneumothorax and hemothorax are considered unusual adverse consequences of IJV cannulation, but with an incidence of 1.3% in a large meta-analysis, statistically the same as 1.5% found for subclavian puncture [76]. It usually results from a skin puncture too close to the clavicle or, rarely, from other causes [56]. Pneumothorax may be complicated by heme, infusion of intravenous fluid, or tension.
An extraordinary number of case reports indicate that any complication from IJV catheterization is possible, even the intrathecal insertion of a Swan-Ganz catheter [77]. In reality, this route is reliable, with a low incidence of major complications. Operator experience is not as important a factor as in SV catheterization; the incidence of catheter tip malposition is low, and patient acceptance is high. It is best suited for acute, short-term hemodialysis and for elective or urgent catheterizations in volume-replete patients, especially pulmonary artery catheterizations and insertion of temporary transvenous pacemakers. It is not the preferred site during airway emergencies, for parenteral nutrition, or for long-term catheterization because infectious complications are higher with IJV compared to SCV catheterizations.
External Jugular Vein Approach
Motivated by the search for a “golden route”; [78], Nordlund and Thoren [79] performed EJV catheterization and advocated a more extensive use of this approach. In 1974, Blitt et al. [80] described a technique of CVC via the EJV employing a J-wire. Although the success rate of this route is lower than with the IJV, a “central”; venipuncture is avoided, and in selected cases catheterization via the EJV remains an excellent alternative. The main advantages to the EJV route for CVC are that it is part of the surface anatomy, it may be cannulated in the presence of clotting abnormalities, and the risk of pneumothorax is all but eliminated. The main disadvantage is the unpredictability of passage of the catheter to the central compartment. We rarely use this approach anymore, primarily because of greater experience with the IJV and SV in patients with coagulopathy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








