LUMBAR PUNCTURE
Lumbar puncture is considered the gold standard diagnostic procedure to assist clinicians with the evaluation of subarachnoid hemorrhage, meningitis, and other neurologic conditions. Anxiolytics, such as benzodiazepines, may be administered to improve patient comfort, relaxation, and cooperation. Contraindications to lumbar puncture are listed in Table 175-1.
Skin infection near the site of lumbar puncture CNS lesion causing increased intracranial pressure, or spinal mass Platelet count <20,000 mm3 is an absolute contraindication; platelet counts >50,000 mm3 are safe for lumbar puncture* International normalized ratio ≥1.5* Administration of unfiltrated heparin or low-molecular-weight heparin in past 24 h* Hemophilia, von Willebrand’s disease, other coagulopathies* Trauma to lumbar vertebrae |
If an anxiolytic is used, give a short-acting agent to avoid clouding subsequent clinical assessment. Antiseptic technique should be strictly observed. Scrub the site with povidone-iodine and allow to dry thoroughly to avoid introduction of the chemical and the subsequent production of chemical arachnoiditis.
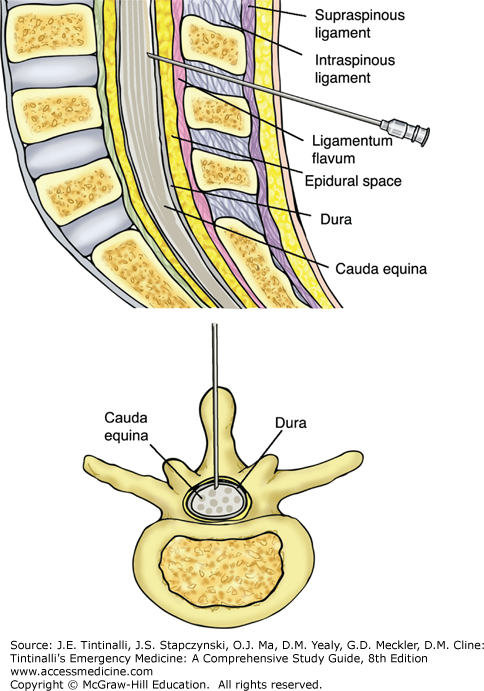
In adults, a transverse line drawn between the iliac crests crosses the spine at the L4 spinous process. The L4-L5 interspace is the most commonly used interspace for lumbar puncture, although one can also select the L3-L4 interspace. Palpate the L4-L5 interspace while the patient is curled as tightly as possible in a fetal position. Alternatively, the patient may be seated on the edge of a bed or cart leaning over a tray stand. This latter technique is particularly useful when landmarks are uncertain due to body habitus.
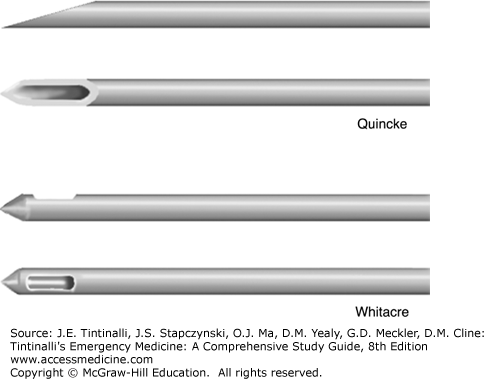
Use a 31/2-inch atraumatic 22-gauge needle in adults. Use of a needle larger than 20-gauge may double the incidence of post–lumbar puncture headache compared with a smaller needle. The use of an atraumatic or pencil-point needle (such as a Whitacre or Sprotte needle) is associated with fewer post–lumbar puncture headaches than a conventional cutting needle1,2 (Figure 175-1). Also, a smaller needle size using the stylet is associated with reduced frequency of post–lumbar puncture headaches.1
Assemble all equipment. Then properly position the patient and identify the patient’s L4-L5 interspace. Now put on your sterile gown, mask, and gloves. Next, apply povidone-iodine to the area and let dry. Apply sterile drapes. Anesthetize the skin with 1% lidocaine by raising a skin bleb, then directing the needle toward the umbilicus, and injecting the anesthetic in a fan-shaped area around the proposed lumbar puncture site. Make sure to pull back on the plunger to check for blood return before injecting to avoid intravascular injection. Recheck the patient’s position, making sure the patient is perpendicular to the horizontal and not slanted. Check the needle and make sure that it is the correct size and that the stylet is easily removed and reinserted. Identify that the needle bevel (Quincke needle) or notch (Whitacre needle) is facing the ceiling. Put together the manometer, making sure you know how the stopcocks work. Assuming you are right-handed, identify the interspinous space with your left hand and position the needle at the L4-L5 interspace, parallel to the horizontal, and direct it firmly and slowly toward the patient’s umbilicus. As the needle advances, you may feel a slight “pop,” indicating that you have pierced the ligamentum flavum and dura mater. Remove the stylet, note draining cerebrospinal fluid (CSF), and attach the manometer to measure the opening pressure. After collecting at least 1 mL in each of four tubes, disconnect the manometer and replace the stylet before withdrawing the spinal needle (Figure 175-2). Do not aspirate because slight negative pressure may facilitate herniation.1
If unable to penetrate the subarachnoid space, it is best to withdraw the needle completely, out of the skin, and direct the needle again. In some patients (for example, those who are extremely obese or who have degenerative disease of the spine), it is particularly difficult to successfully perform a lumbar puncture. The use of US guidance or even consultation of a radiologist to perform under fluoroscopy may be necessary.
US imaging of the interspinous processes is superior to palpation with regard to fewer failed attempts.3 A 5- to 10-MHz linear probe is applied in the sagittal plane to identify the dorsal spinous processes, and the midline is then marked with a sterile marking pen. Lumbar puncture is then performed as outlined above.
For results to be meaningful, measure the opening pressure with the patient lying extended on his or her side. Pressures measured with the patient still curled in extreme flexion or sitting may be artificially elevated. Normal pressure is <170 mm H2O. Careful repositioning (straightening the curled patient or helping the seated patient to a lying position on his or her side) can be safely performed with the needle in place. Obtain accurate pressure readings only when the patient is in a calm state if possible. Shouting, crying, or coughing can elevate the pressure falsely.1
Obtain four tubes, each containing at least 1 mL of CSF. Obtain red and white blood cell counts with differential for tubes 1 and 4. Tube 4 may also be used for culture and Gram staining. Tube 2 is sent for determination of protein and glucose levels. Save tube 3 for other studies that may be required. Table 175-2 lists normal CSF findings. See chapters 166, “Spontaneous Subarachnoid and Intracerebral Hemorrhage” and 174, “Central Nervous System and Spinal Infections” for further discussion of CSF interpretation.
| Parameter | Value |
|---|---|
| Opening pressure | 50–170 mm H2O |
| Appearance | Clear (can be clear with up to 400 cells/mm3) |
| Xanthochromia | None |
| Red blood cells | ≤5/mm3 |
| WBCs | ≤5/mm3 (no polymorphonuclear leukocytes) |
| Glucose level | >40 milligrams/dL or 60%–70% of serum glucose level |
| Protein level | <50 milligrams/dL |
| Gram stain and culture results | Negative |
Local discomfort is to be expected; radicular pain indicates that the needle is too lateral, which necessitates repositioning. Spinal hematoma is a particularly ominous complication. The development of severe or persistent back pain after the procedure, radicular pain, new neurologic symptoms, or sphincter disturbance indicates that a spinal hematoma may be present. Most present within the first 6 hours.1 Management includes MRI of the spine and neurosurgical consultation.
Post–lumbar puncture headache is the most common complication of lumbar puncture. The cause is thought to be continuous CSF leakage from the dural puncture site. Traction on bridging vessels, dura, and nerves causes headache. Post–lumbar puncture headache usually begins 24 to 48 hours after the procedure and is located in the frontal or occipital area. It is pressure-like and throbbing, with variable intensity. It is intensified when the patient is sitting or standing upright and with Valsalva maneuvers such as coughing. The headache improves or resolves when the patient is supine. It can be associated with nausea, vomiting, and even vertigo and tinnitus. Risk factors for post–lumbar puncture headache include use of a large needle size (>22 gauge), use of a cutting (Quincke) needle, multiple attempts, and failure to replace the stylet when withdrawing the needle.1,2 Post–lumbar puncture headache does not seem to be related to the opening pressure, the volume of CSF removed, or bed rest after the procedure. Postprocedural bed rest does not prevent this complication.4 Diagnosis is clinical. Most headaches resolve with rest in the supine position, maintenance of hydration, and administration of antiemetics and analgesics.1 IV caffeine (500 milligrams IV of caffeine sodium benzoate) is commonly administered, but whether or not it improves headache is controversial.5 Persistent headache (>24 hours) can be treated with an epidural blood patch. In this procedure, usually performed by an anesthesiologist, a needle is introduced into the epidural space, and 20 to 30 mL of the patient’s blood is injected into the epidural space.
CEREBROSPINAL FLUID SHUNTS
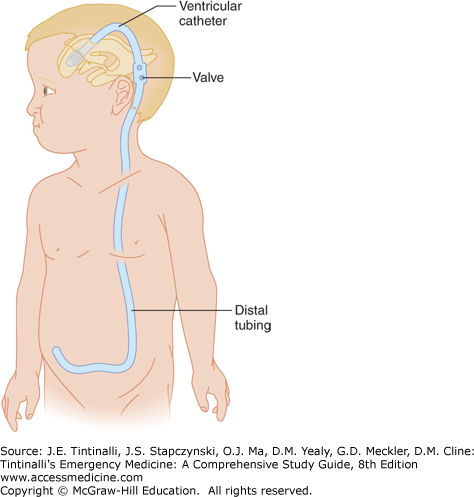
Mechanical shunting is the primary treatment for hydrocephalus. Placement of a CSF shunt is the most common pediatric neurosurgical procedure performed in the United States. It is also the neurosurgical procedure with the highest incidence of postoperative complications.6 Many types of CSF shunt systems exist (Figures 175-3, 175-4, and 175-5). Most systems consist of three components, beginning with a silastic tube passed into the ventricle via a burr hole. This tubing is tunneled subcutaneously to a valve chamber. The valve chamber, the second component, establishes a pressure gradient that ensures drainage of fluid away from the ventricle. The valve chamber, or in some cases a separate reservoir, allows access to the shunt system for patency testing, pressure measurement, CSF sampling, medication injection (e.g., chemotherapy, antibiotics), or contrast administration. Distal tubing, which is the third component, connects the valve chamber to a drainage point. The most common drainage site is the peritoneal cavity. Other drainage sites include the right atrium, gallbladder, pleural cavity, and ureter.
Programmable shunt valves allow for easier control of flow rates, which is particularly useful in previously difficult cases that required frequent adjustments. The valve can be adjusted and tested using a locator and indicator tool to determine the pressure programmed into the valve. An adjustment tool can then be used to increase or decrease the valve’s pressure or performance as needed. Typical nonadjusTable pressure-type valves are available with low, medium, and high settings. These valves open at a pressure gradient of 2 to 4, 4 to 6, and 8 to 10 cm H2O for the low, medium, and high settings, respectively. The adjusTable valves typically have five preset pressure settings that can be selected or adjusted as clinically warranted. The patient (or family) should have been given a card that documents initial settings and any subsequent adjustments. Pressure setting can also be confirmed by radiography using the radiopaque dials on the valve.
Exposure to strong magnetic fields and some MRI units can change the valve pressure setting, so all patients should have the setting verified after any exposure to strong magnetic fields. Controversy still exists as to whether programmable valves offer an advantage over traditional valves in reducing the number of revisions and extending shunt life.7,8,9
Shunt malfunctions are the most common complications encountered with CSF shunts. Shunt malfunction can be due to obstruction, mechanical failure, overdrainage, loculation of ventricles, or abdominal complications.
Obstruction is the most common type of shunt malfunction. The most frequent location of obstruction is the proximal tubing, followed by the distal tubing, and then the valve chamber. Proximal obstructions usually occur within the first years after shunt insertion. Table 175-3 lists the common causes of CSF shunt obstruction.
Distal obstruction is the most frequently encountered obstruction in shunts in place for >2 years.6 Shunt obstruction usually manifests with signs and symptoms of increased intracranial pressure. Infants generally present with vomiting, irritability, and a bulging fontanelle.10 Older children and adults may present with cephalgia, nausea, vomiting, lethargy, ataxia, and cranial nerve palsies.10
Mechanical failure of shunts can be secondary to fracture, disconnection, migration, or misplacement. Typically, fractures appear in distal tubing many years after shunt placement; this is due to both degradation of tubing and stress from the growth of the patient.6 The most common location for a fracture is along the clavicle or lower ribs.6 Patients present with mild symptoms of increased intracranial pressure. Local symptoms of pain, mild erythema, and edema over the affected area are not uncommon. In fact, it not unusual for a fracture to be found incidentally because the shunt tract often serves as a conduit between the fractured segments.6 Disconnection often occurs shortly after insertion and manifests as increased intracranial pressure and fluid at the skin site around the disconnection. Migration occurs when a properly placed catheter migrates to a position in which drainage is compromised partially or completely. Misplacement entails the placement of the catheter into brain parenchyma, the choroid plexus, or the temporal horns; it usually manifests postoperatively with evidence of failure.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree