CHAPTER 45
Celiac Plexus Block Using CT Guidance
INDICATIONS
The major indications for celiac plexus block (CPB) include abdominal pain which is nonresponsive to less aggressive analgesic interventions and which emanates from:
• The liver
• Gallbladder
• Omentum
• Mesentery
• The alimentary tract from the stomach to the transverse large colon,3 which is of a visceral and not a somatic nature
These indications include pathology that is both of a benign as well as a malignant etiology.
Neurolytic CPB is typically reserved for cancer-related pain, while it is more common that local anesthetic and occasionally steroid injections are reserved for benign type of pain. Following performance of CPB using 50% to 100% alcohol for malignant pain, an early meta-analysis found that pain relief was “good or excellent” in 89% of 989 patients for the first 2 weeks following the block.4
PREOPERATIVE CONSIDERATIONS
• Determine the etiology of painful processes of the abdomen. It is essential to rule out nonvisceral causes.
• Perform somatic blocks prior to undertaking CPB.
• Intercostal nerve blocks (ICNB) may be one such approach to using somatic blocks in an effort to differentiate somatic from visceral etiologies.
• Judicious use of local anesthetic agents for ICNB may help determine whether or not a pathologic process has primarily a somatic origin versus a sympathetic one.
RELEVANT ANATOMY
• Several of the abdominal viscera receive innervation through the celiac plexus.
• Sympathetic innervation is derived from the anterolateral horn of the spinal cord as axons from T5 to T12 leave the spinal cord with ventral nerve roots to join white rami communicantes (WRC) in route to the sympathetic chain.
• These axons do not synapse in sympathetic chain; instead, they pass through the chain to synapse at distal sites.
• The celiac plexus includes several ganglia, these being the celiac, aortic, renal, and superior mesenteric ganglia.
• Postganglionic nerves accompany blood vessels to visceral structures.
• Preganglionic fibers from T5 to T9 travel caudally from the sympathetic chain along the lateral and anterolateral aspects of the respective vertebral bodies.
• The greater splanchnic nerve arises from the T9 and T10 levels.
• The celiac plexus (Figure 45-1) sits anterior to the aorta, epigastrium, and crus of the diaphragm. Fibers comprising the celiac plexus arise from preganglionic splanchnic nerves, parasympathetic preganglionic nerves from the vagus nerve (CN X), some sensory nerves from the phrenic and vagus nerves, and postganglionic sympathetic fibers.
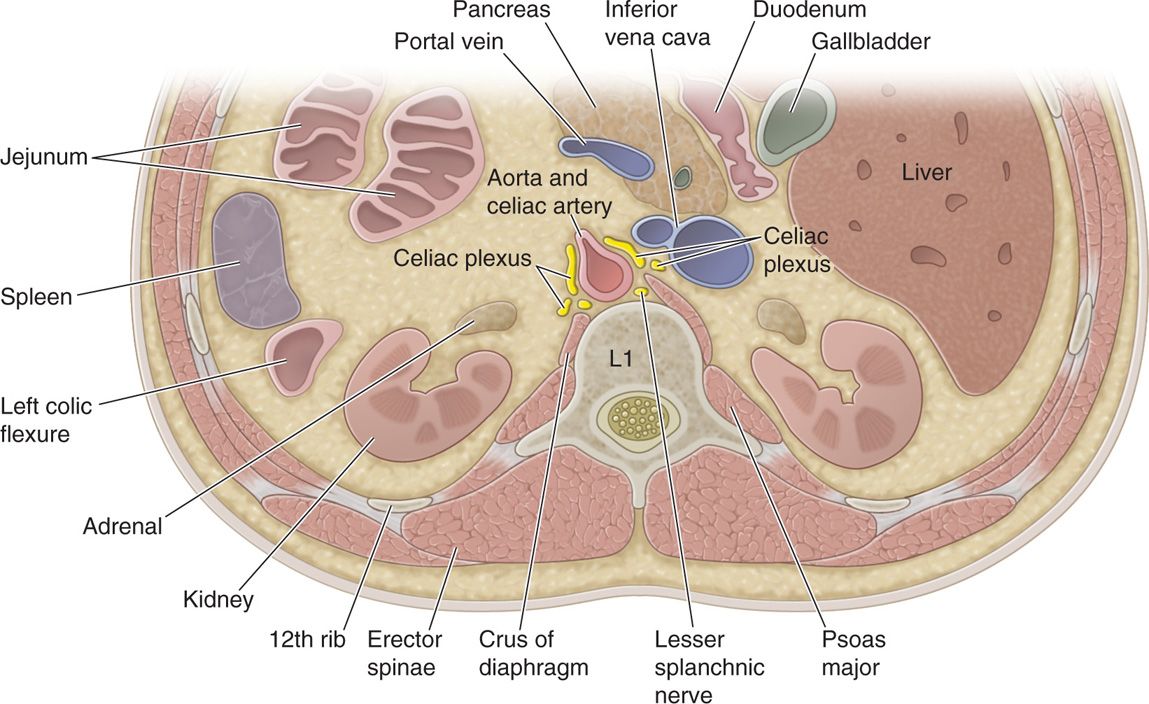
Figure 45-1. Anatomy of the celiac plexus and surrounding visceral structures.
• Complete sympathetic denervation of the gastrointestinal tract, such as may occur following CPB renders an individual likely to develop increased peristalsis due to unopposed parasympathetic nervous system activity.
The celiac plexus is in actuality the confluence of three distinct pairs of ganglia; the celiac, superior mesenteric, and aorticorenal ganglia, respectively. Afferent nociceptive fibers are the identified targets of the CPB. The CPB is useful as a diagnostic and as a therapeutic tool for managing pain of diverse intraabdominal etiologies including both benign as well as malignant causes.
PREOPERATIVE CONSIDERATIONS
• Obtain an informed, written consent.
• Confirm the absence of contrast allergies or anticoagulant medications.
• It is useful to refer to standard references such as those published by the American Society of Regional Anesthesia (ASRA) concerning anticoagulants and regional anesthesia prior to commencing to determine the suitability of prospective block candidates prior to proceeding.
• Standard American Society of Anesthesiologists (ASA) monitors are applied and baseline vital signs are assessed and documented.
• An intravenous cannula should be placed for the purposes of administering intravenous fluids and supportive drugs in the event of either an inadvertent event or following the expected orthostatic hypotension resulting from a successful block.
Selection of Needles, Medications, and Equipment
• 20 or 22-gauge 12- or 18-cm spinal type needle (Quincke)
• Water-soluble, iodine-based contrast
• Local anesthetics (0.2% ropivacaine; 0.125% bupivacaine; 0.5% lidocaine)
• CT scanner or fluoroscopy equipment
• Endoscopic ultrasound equipment (for endoscopic technique only)
INTRAOPERATIVE TECHNICAL STEPS (FIGURES 45-2 TO 45-17)
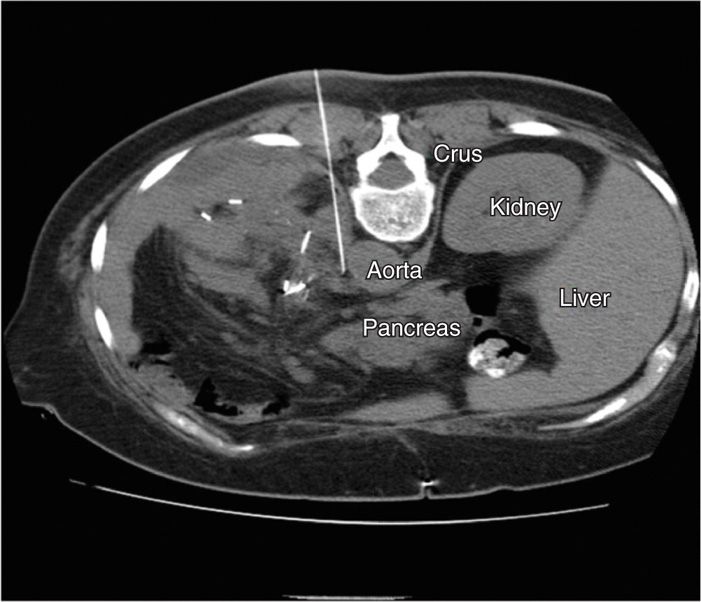
Figure 45-2. Posterior CPB. Axial CT scan slice at the T12-L1 level demonstrating a left-sided approaching needle in a para-aortic approach to single-injection CPB. Technique demonstrated is a trans-crural approach. (Figure courtesy of Kenneth D. Candido, MD.)
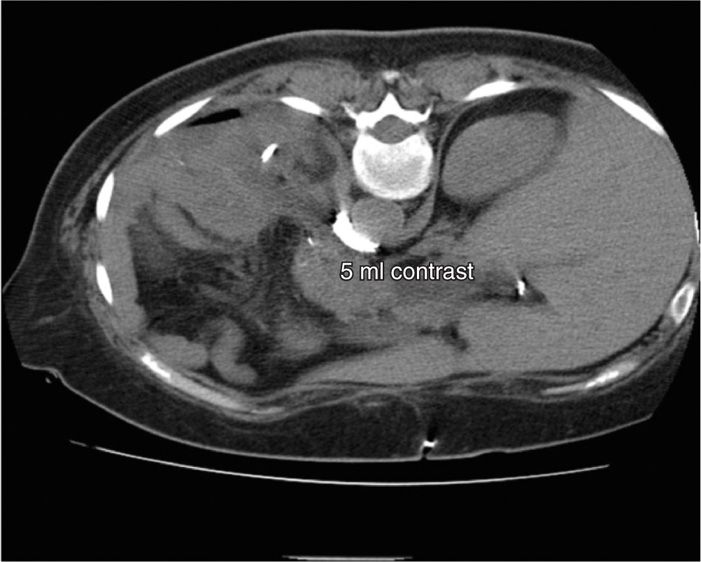
Figure 45-3. Posterior CPB. Same patient as above. 5 mL of iodine-based, water-soluble contrast has been injected through the needle, with the beginnings of a para-aortic spread being identified. (Figure courtesy of Kenneth D. Candido, MD.)
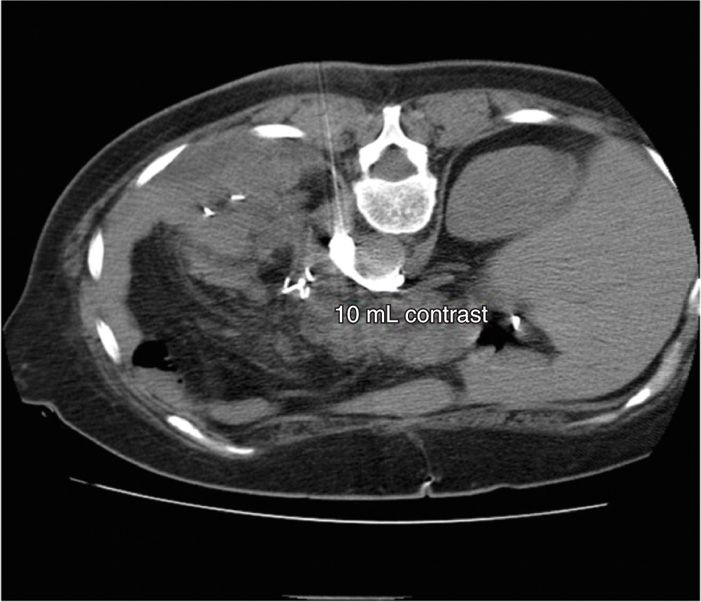
Figure 45-4. Posterior CPB. Same patient as above. 10 mL of contrast is now shown beginning to extend circumferentially toward the right side, in addition to the left side. (Figure courtesy of Kenneth D. Candido, MD.)
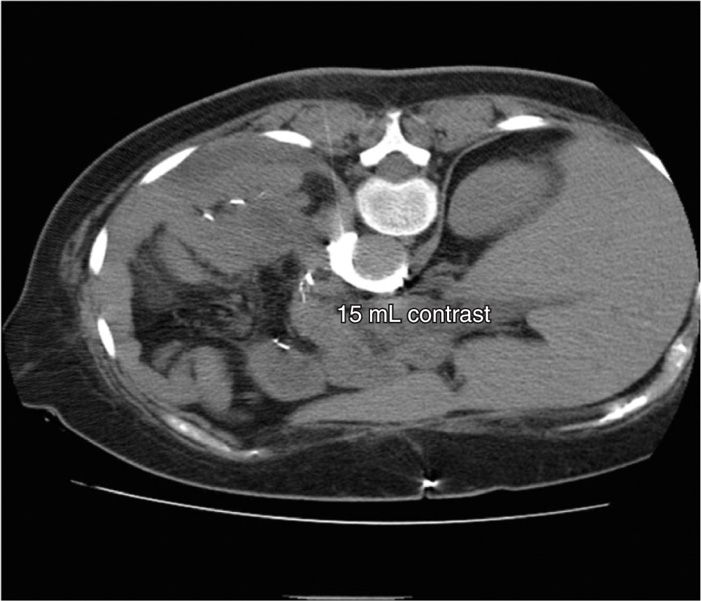
Figure 45-5. Posterior CPB. Same patient as above. Spread of 15 mL of contrast moves deeper in the para-aortic space bilaterally. (Figure courtesy of Kenneth D. Candido, MD.)
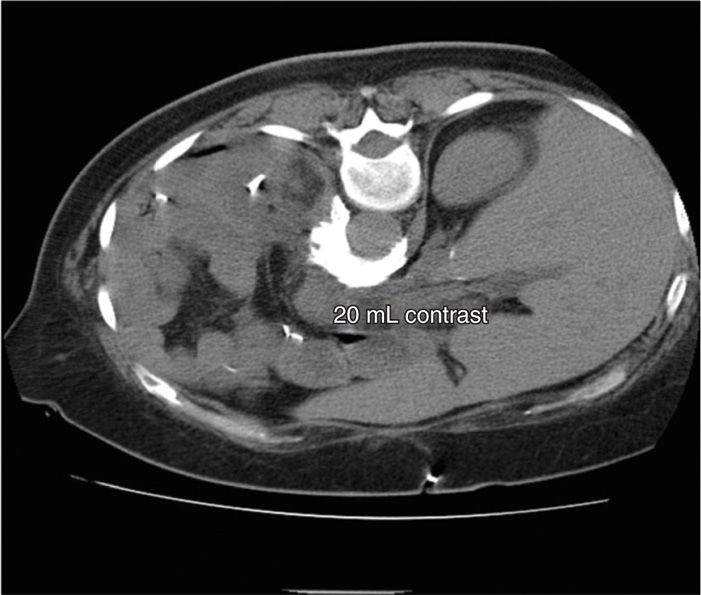
Figure 45-6. Posterior CPB. Same patient as above. 20 mL of contrast demonstrate bilateral spread in a para-aortic fashion. (Figure courtesy of Kenneth D. Candido, MD.)
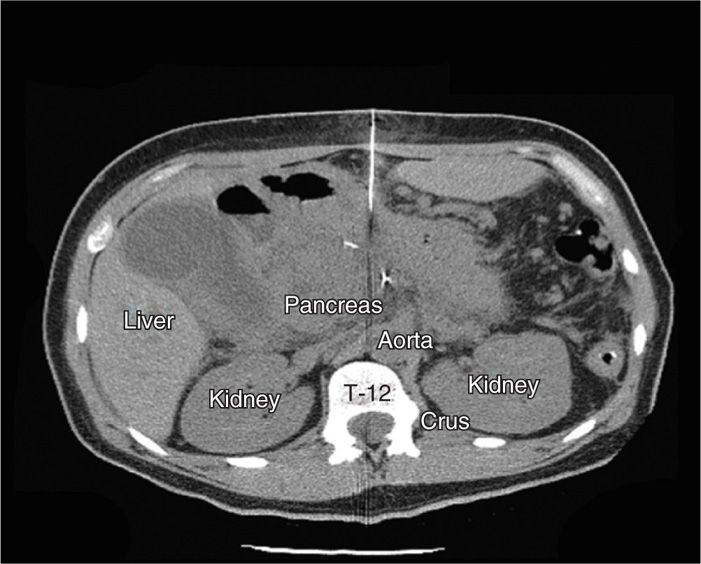
Figure 45-7. Anterior CPB. Needle advancement, midline at T12 level. Bright, hyperintense areas in abdomen represent recent surgical clips from an “open-and-shut” laparotomy performed 6 days earlier. (Figure courtesy of Kenneth D. Candido, MD.)
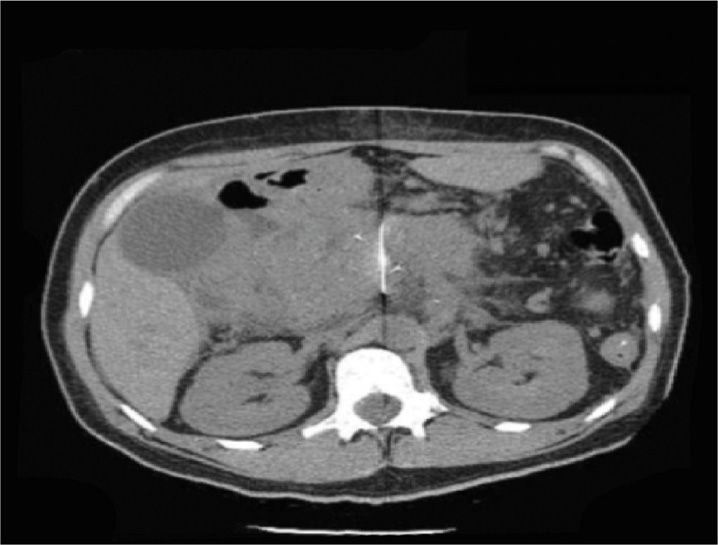
Figure 45-8. Anterior CPB. Same patient as above. Needle approaches the aorta, midline at T12 level. (Figure courtesy of Kenneth D. Candido, MD.)
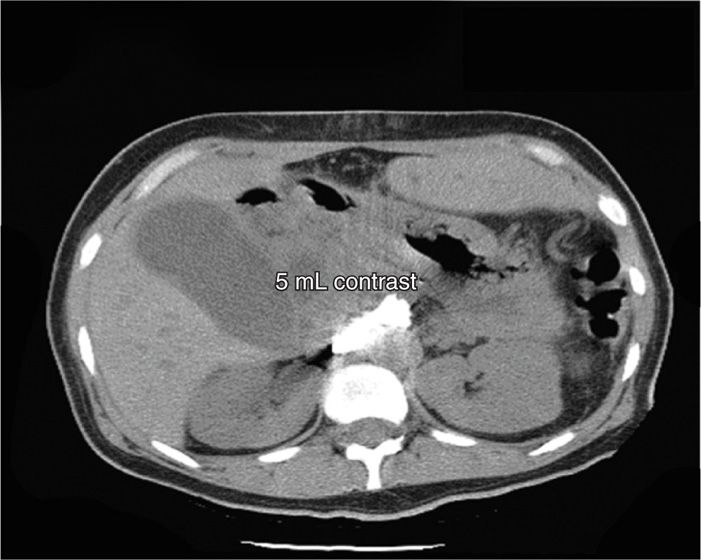
Figure 45-9. Anterior CPB. Same patient as above. 5 mL of contrast spreading ventral to the aorta in the suspected location of the bilaterally paired celiac plexus. (Figure courtesy of Kenneth D. Candido, MD.)
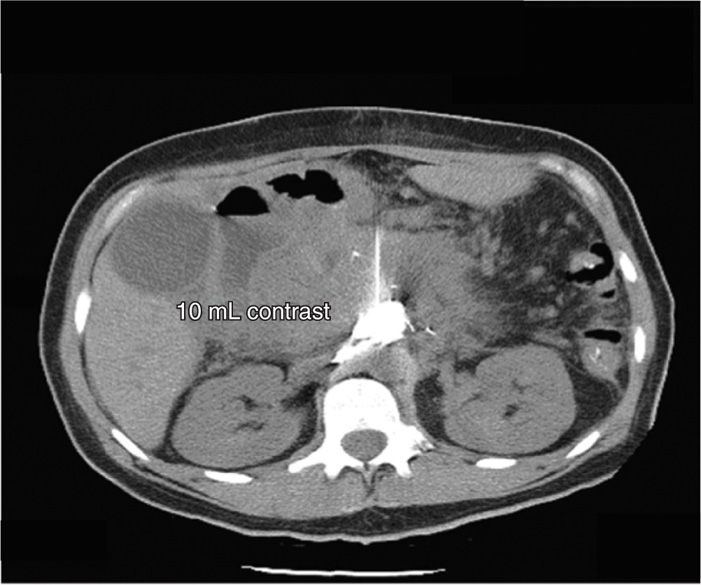
Figure 45-10. Anterior CPB. Same patient as above. 10 mL of contrast begins to spread bilaterally. Extensive scarring in the abdomen prevents material from extending posteriorly toward the vertebral body of T12. (Figure courtesy of Kenneth D. Candido, MD.)
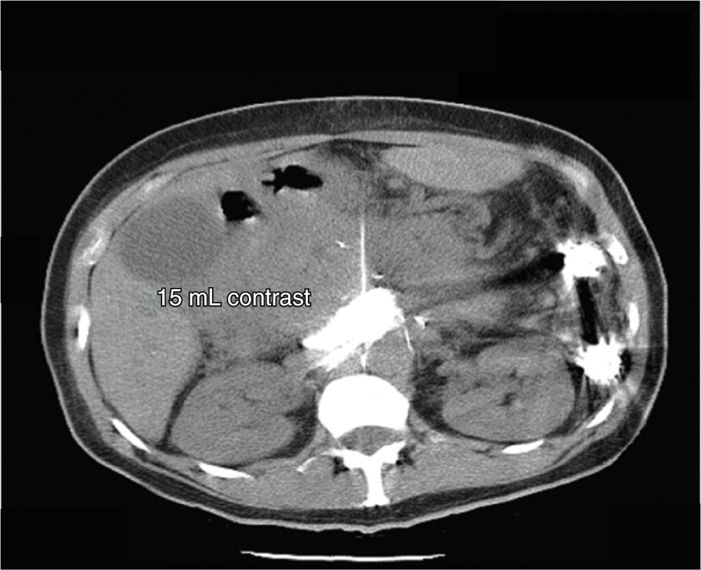
Figure 45-11. Anterior CPB. Same patient as above. Now, with the injection of 15 mL of contrast, the contrast material adheres to the anterior and lateral walls of the abdominal aorta, inhibited only by the intraabdominal scarring from prior surgical interventions. (Figure courtesy of Kenneth D. Candido, MD.)
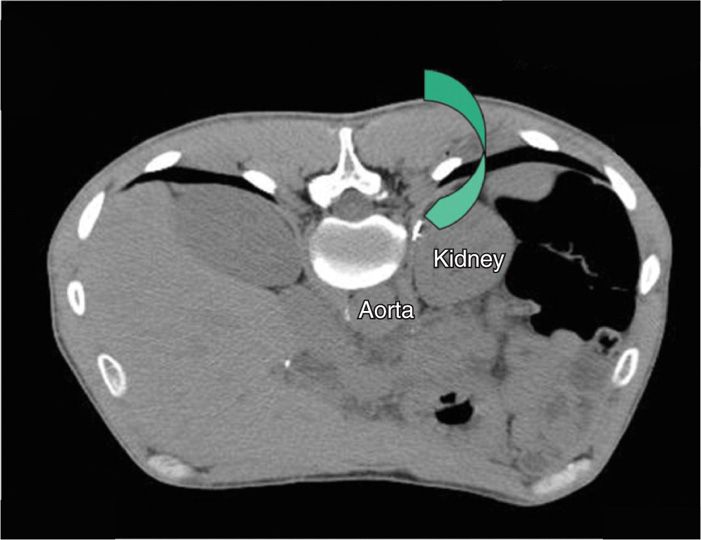
Figure 45-12. Transaortic CPB. Green arrow points to needle tip advancing medial to the left kidney and lateral to the T12 vertebral body. (Figure courtesy of Kenneth D. Candido, MD.)
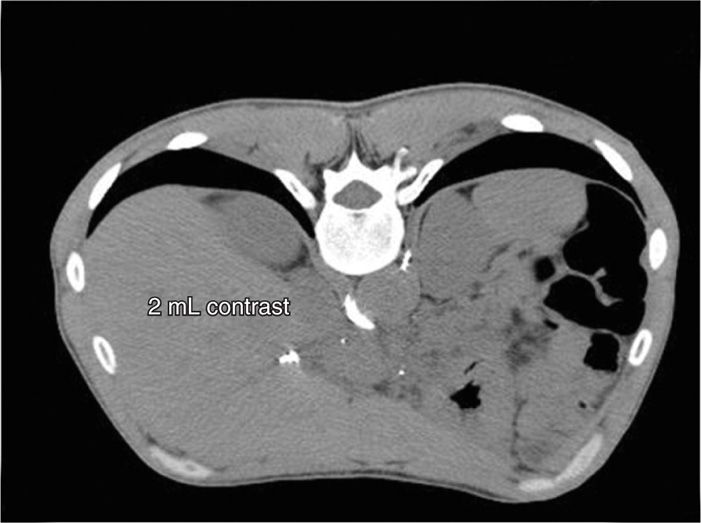
Figure 45-13. Transaortic CPB. 2 mL of contrast has been injected after stylet removal demonstrated bright red backflow of blood. (Figure courtesy of Kenneth D. Candido, MD.)
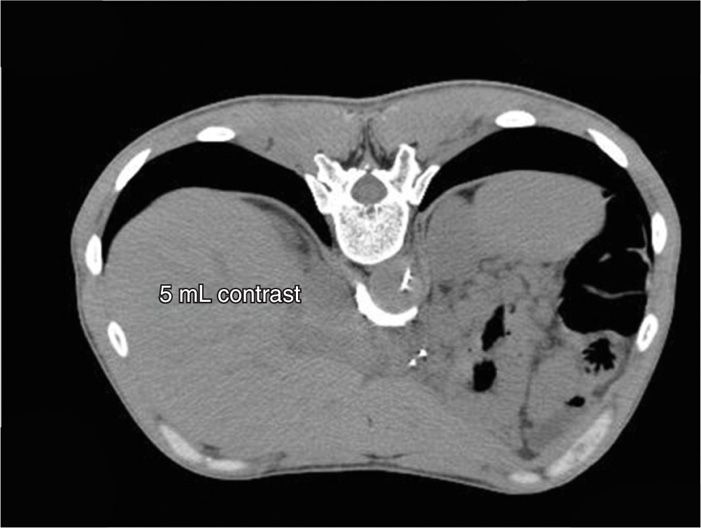
Figure 45-14. Transaortic CPB. 5 mL of contrast have been injected, demonstrating a crescent-shaped, half-moon, para-aortic spread beginning to spread bilaterally. (Figure courtesy of Kenneth D. Candido, MD.)
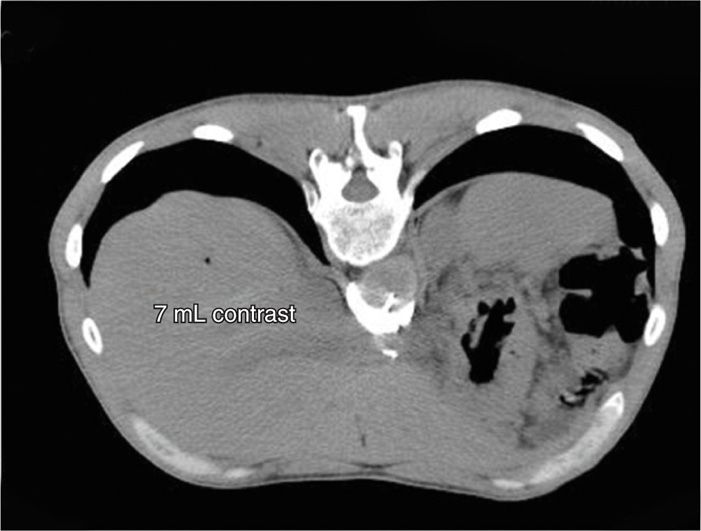
Figure 45-15: Transaortic CPB. Additional 2 mL of contrast spreading further bilaterally. (Figure courtesy of Kenneth D. Candido, MD.)
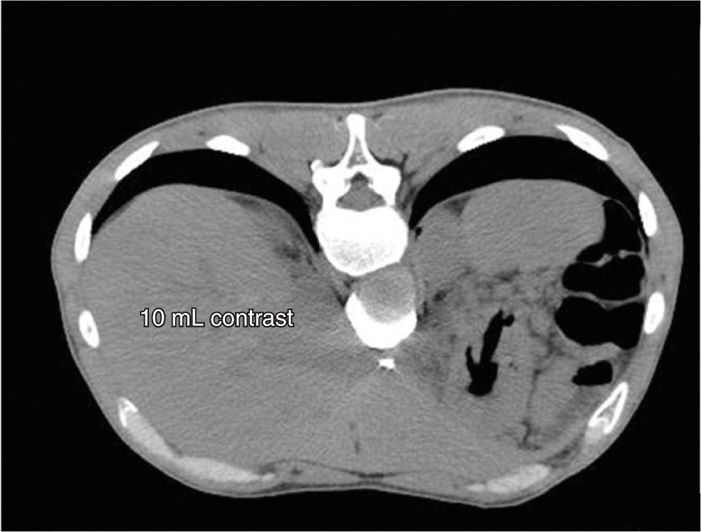
Figure 45-16. Transaortic CPB. A total of 10 mL of contrast has now been injected, demonstrating excellent spread bilaterally, in a para-aortic fashion, covering the suspected distribution of the celiac plexus. (Figure courtesy of Kenneth D. Candido, MD.)
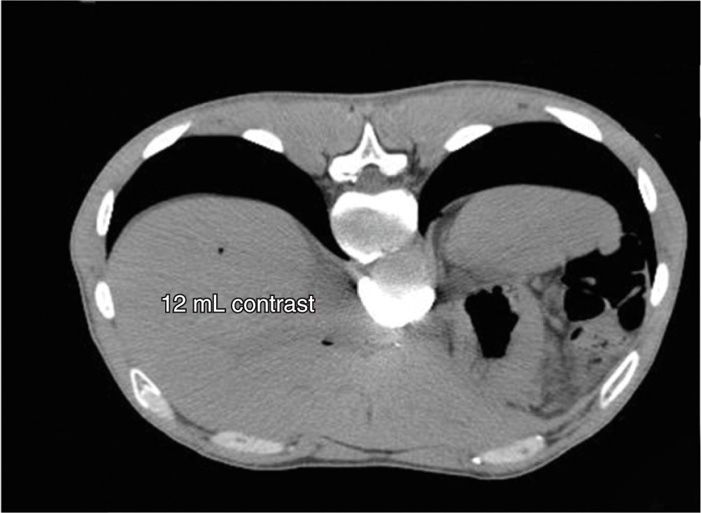
Figure 45-17. Transaortic CPB. A total of 12 mL have been injected, demonstrated a spread equivalent to the posterior approach technique shown above which required almost 20 mL to completely encapsulate the abdominal aorta and extend to the bilateral celiac plexus. (Figure courtesy of Kenneth D. Candido, MD.)
CPB may be undertaken using one of a variety of approaches, including those which utilize fluoroscopic or computed tomography (CT) guidance and those for which ultrasound or endoscopy is determined to be appropriate as an adjunct to identifying the relevant anatomy. In 2012, it is probably not feasible to consider performing CPB absent some type of imaging technique. The celiac plexus may be approached using 1 of the 3 basic anatomical techniques:
1. Posterior para-aortic (Figures 45-2 to 45-6)
2. Anterior2 (Figures 45-7 to 45-11)
3. Posterior transaortic (Figures 45-12 to 45-17)
Posterior Para-Aortic Approach
• The patient is placed in the prone position.
![]() Placing a pillow or bolster beneath the abdomen may help reduce the normal lumbar spinal lordotic curve.
Placing a pillow or bolster beneath the abdomen may help reduce the normal lumbar spinal lordotic curve.
• A full surgical sterile skin preparation and draping is next undertaken.
• When imaging is used, it is imperative to obtain a scout film of the lower thoracic and upper lumbar spinal areas, seeking to identify the T12 and L1 vertebral bodies by visualizing their respective spinous processes.
• The inferior border of the 12th rib is identified and marked using a sterile marking skin pen.
• A local anesthetic skin wheal is raised using a hypodermic needle and a small-gauge needle, approximately 7 to 8 cm from the anatomical midline, at the inferior border of the 12th rib.
• Next, a 20- or 22-gauge, 12- or 18-cm spinal type needle (Quincke) is advanced from posterior to anterior toward the ventral surface of the T12-L1 intervertebral space, as defined by the intervertebral disc, taking care not to penetrate the actual disc.
![]() The goal of needle advancement is to remain in close proximity to the lateral aspect of the vertebral bodies during the entire course of needle penetration towards the target.
The goal of needle advancement is to remain in close proximity to the lateral aspect of the vertebral bodies during the entire course of needle penetration towards the target.
![]() Starting out farther than 8 cm from the anatomical midline poses a risk of renal injury due to penetration by the advancing needle tip.
Starting out farther than 8 cm from the anatomical midline poses a risk of renal injury due to penetration by the advancing needle tip.
![]() If the starting point is beneath the lateral tip of the 11th, and not the 12th rib, there is a distinct risk of producing a pneumothorax.
If the starting point is beneath the lateral tip of the 11th, and not the 12th rib, there is a distinct risk of producing a pneumothorax.
![]() With CT-guided techniques, of course, many of these same concerns are rendered moot, as the anatomy is more easily reconciled (Figures 45-2 to 45-17).
With CT-guided techniques, of course, many of these same concerns are rendered moot, as the anatomy is more easily reconciled (Figures 45-2 to 45-17).
• The needle (a left-sided approach may be attempted using a single-needle, or alternatively, bilateral needles may be placed if there is not a satisfactory spread of contrast using a unilateral approach) is advanced toward the T12-L1 interspace using CT or fluoroscopic imaging.
![]() A left-sided approach has been demonstrated to more reliably block the celiac plexus when a single-needle is utilized.5
A left-sided approach has been demonstrated to more reliably block the celiac plexus when a single-needle is utilized.5
![]() In the viewpoint of these authors, who have performed >500 CPBs, it has been decidedly the rare occasion that more than a single needle has ever been necessary to successfully attain bilateral contrast spread using the described technique(s).
In the viewpoint of these authors, who have performed >500 CPBs, it has been decidedly the rare occasion that more than a single needle has ever been necessary to successfully attain bilateral contrast spread using the described technique(s).
• If the needles are directed more cephalad, toward the spinous processes of T12 or T11, it is likely that a splanchnic and not a celiac plexus block is in actuality being performed.6
• Penetration or nonpenetration of the crus of the ipsilateral diaphragm while performing a posterior approach to CPB determines whether the block is a “true” CPB or whether in fact it is a splanchnic nerve block (SNB).7
![]() If the needle is posterior to the crus, the block is an SNB; anterior to the crus is a true CPB (Figure 45-4).
If the needle is posterior to the crus, the block is an SNB; anterior to the crus is a true CPB (Figure 45-4).
• The optimal angle for needle insertion varies using a left-sided approach but has been calculated to be between 18 degree (at T12) and 19 degree (at L1).8
• Once the vertebral body has been contacted, the needle is advanced laterally until it slips off lateral surface of the bone.
![]() It helps to place a curve at the distal tip of the block needle so that it may be steered off the bone without the requirement to actually withdraw it and reinsert it to bypass the lateral vertebral margin.
It helps to place a curve at the distal tip of the block needle so that it may be steered off the bone without the requirement to actually withdraw it and reinsert it to bypass the lateral vertebral margin.
![]() Alternatively, avoiding bony contact altogether may reduce patient discomfort during performance of CPB.9
Alternatively, avoiding bony contact altogether may reduce patient discomfort during performance of CPB.9
• Once the needle passes through the psoas fascia, a distinct “pop” may be appreciated, although this is by no means noted for every case. It is imperative to recall that the aorta will be found on the left of the vertebral body (Figure 45-1) while the inferior vena cava will be on the right side.
• Once the needle(s) are in a satisfactory anatomical position, the next step is to perform some type of contrast injection when CT or fluoroscopic imaging is being used.
![]() When ultrasound is the selected imaging technique of choice, it is just as imperative to identify the relevant vascular structures which identify the location of the celiac plexus, as the structure itself is not likely to be visible even when using low-frequency curvilinear probes needed for their greater depth of penetration for such a block.
When ultrasound is the selected imaging technique of choice, it is just as imperative to identify the relevant vascular structures which identify the location of the celiac plexus, as the structure itself is not likely to be visible even when using low-frequency curvilinear probes needed for their greater depth of penetration for such a block.
• A water-soluble, iodine-based contrast agent is next injected, the volume of which is determined by a real-time assessment of the spread of the agent.
• The present authors suggest using a volume commensurate with bilateral, para-aortic spread (under CT guidance; Figures 45-6, 45-11, 45-16, 45-17).
• Importantly, the actual volume of local anesthetic used for the analgesic phase of the block should be determined largely based upon how the contrast spreads, as it makes no sense to use predetermined or “cook-book” volumes of solutions in disregard of the anatomical confines identified using the aforementioned imaging modalities.10–12
Anterior Para-Aortic Approach2
• An anterior approach may be selected, especially for individuals who cannot or who will not be able to lie prone. A common scenario of this nature would be someone who has recently undergone a surgical procedure who has significant abdominal pain when assuming the prone position.
• Advantages to using the anterior approach include the ability to advance a single block needle from anterior to posterior toward the abdominal aorta.
• Disadvantages include the requirement to insert that needle directly through the abdominal viscera as the target of the block cannot be approached without doing so.
• The use of CT imaging (Figures 45-7 to 45-11) enhances an appreciation of the targeted and nontargeted structures directly in the pathway of the advancing block needle.
After the usual skin disinfection and after the preparation of the patient as described above for the posterior approach has been undertaken, a local anesthetic skin wheal is placed anterior to the T12 vertebral body as seen on imaging, followed by the insertion and advancement of a 20- or 22-gauge 12- or 18-cm spinal type needle (Quincke) directed toward the abdominal aorta.
• Contrast use as described above is then undertaken.
• Recent evidence suggest that a single-needle anterior CPB may not effectively spread contrast (and neurolytic agent) into all four quadrants (upper right and left and lower right and left), which may also affect the resultant analgesic response to neurolysis.2
Posterior Trans-Aortic Approach
• Occasionally during the performance of a posterior CPB, whether intentionally or unintentionally, the abdominal aorta will be punctured (Figures 45-12 to 45-17).
• It is obviously much more likely that this situation will be identified when CT imaging is used than when standard (nondigital subtraction angiography-DSA) imaging is undertaken.
• When the needle stylet is withdrawn under such circumstances, a few drops of bright red blood may be identified at the hub of the needle, but seeking a “gush” of blood is typically pointless as this rarely occurs. This is due to the characteristics of fluid flow in these longer, fine needles (20- and 22-gauge) typically used for CPB as determined by the Hagen-Poiseuille equation which states that:
ΔP = 8 μLQ/πr4
where:
ΔP is the pressure drop from the aorta to the hub of the needle
L is the length of the block needle
μ is the dynamic viscosity of blood
Q is the volumetric flow rate
r is the radius of the nerve block needle
π is the mathematical constant 3.14
• If the vessel is penetrated, it is prudent to advance the needle until blood flow from the hub ceases.
• Under these circumstances, the procedure is then undertaken in a manner virtually identical to that described above for posterior para-aortic CPB including the injection of contrast followed by a dilute local anesthetic in concentrations sufficient to block the sympathetic nerves while sparing sensory and motor nerves (ie, 0.2% ropivacaine, 0.25% bupivacaine, 1.0% lidocaine, for example).
ENDOSCOPIC TECHNIQUES13–22
Recently, interest has focused on using nonfluoroscopy and non-CT guided approaches to performing CPB. Techniques have been described using endoscopic ultrasound equipment (EUS) to avoid radiation exposure and are purported to reduce complications such as pneumothorax and paraplegia from the posterior approaches.13 Some physicians believe that EUS should be considered first-line therapy in patients suffering from pancreatic cancer who require CPB.13
• Endoscopic ultrasound approaches to CPB have been performed for at least the past 16 years.13
• A linear array ultrasound endoscope and a prototype needle-catheter were transgastrically positioned to inject the plexus using bupivacaine and 98% dehydrated absolute alcohol in 30 patients. 88% of patients had persistent improvement in pain scores and used less opioid analgesics at the 12-week follow-up evaluation.14
• A prospective evaluation of the technique performed in 58 patients using the same approach with a 6-month follow-up. 78% of patients had a reduction in pain scores associated with a reduction in narcotic consumption. Only 5 episodes of abdominal pain and no major complications were identified.15
• A meta-analysis of EUS-guided CPB to manage chronic abdominal pain associated with chronic pancreatitis or pancreatic cancer reported that EUS-guided CPB was 51.46% effective in managing chronic abdominal pain from pancreatitis but was 72.54% effective for managing the pain of pancreatic cancer.16
• While the technique of EUS-guided CPB continues to evolve and mature,18 there is need for controlled studies to compare this approach with alternative imaging techniques, as all the evidence is derived from observational and uncontrolled case series.19
• EUS has been touted as being able to identify the actual celiac ganglia comprising the celiac plexus.
• It is as a hypoechoic, small, and either multilobulated or confluent series of small spheres with hypoechoic bands of tissue.20
• Visualization of the ganglia is noted to be the single-most significant predictor of successful block.21
• Patients with visible ganglia are 15 times more likely to respond favorably than those wherein the ganglia are not visible.21
• In one study comparing EUS with fluoroscopically-guided CPB using 10 mL of 0.25% bupivacaine and 40 mg of Triamcinolone, in patients suffering from chronic pancreatitis, 70% of EUS patients showed an improvement in pain scores versus 30% in the fluoroscopy group.22 However, by the end of 24 weeks, almost all patients had returned to their baseline levels of pain.22
POSTPROCEDURE CONSIDERATIONS
Complications occurred in 2% of patients, yet the studies included 32% of cases performed without the benefit of any imaging whatsoever.4 When neurolytic CPB is performed for pancreatic cancer, it appears that cancers of the head of the pancreas respond more favorably than those associated with lesions elsewhere in the pancreas.23 Not only is neurolytic CPB favorable as an analgesic adjuvant in pancreatic cancer pain, it may also elevate patient’s mood, reduce pain interference with activity, and possibly increase life expectancy by some unknown mechanism.24
Monitoring of Potential Complications25–32
Nevertheless, it should be pointed out that there is the potential for severe and often grave consequences of CPB which may occur even in the face of image-guidance and expertly performed procedures. Among the known risks, the following have been reported25,26:
• Hypotension
• Paresthesias of lumbar somatic nerves
• Intravascular injection (arterial or venous)
• Lumbar somatic nerve root injury
• Subarachnoid or epidural injection
• Diarrhea
• Renal injury
• Paraplegia
• Pneumothorax
• Chylothorax
• Vascular thrombosis or embolism
• Vascular trauma
• Perforation of cysts and tumors
• Intradiscal injections and disciitis
• Injection into the psoas muscle
• Abscess
• Peritonitis
• Retroperitoneal hematoma
• Ureteral injury
• Pain both during or after the procedure
• Failure to derive an analgesic response
CLINICAL PEARLS AND PITFALLS
• Celiac plexus block is a valuable adjunctive therapy for managing abdominal pain associated with a visceral pathology.
• Pain reduction is consistently demonstrated following successful block, and there is the potential for reducing opioid consumption and opioid-related side effects and complications.
• Whether or not the implementation of neurolysis using this technique enhances or prolongs life expectancy in terminal cancer, however, remains to be seen.
• A variety of imaging techniques and approaches to the plexus are available, each of which boasts some advantage over the others.
• The clinician undertaking CPB should be well-versed in not only abdominal and retroperitoneal anatomy, but also in the anatomy and physiology of spinal nerves and the spinal cord, and should be prepared to deal with the many expected and occasionally unexpected sequelae following CPB.
• It should be appreciated that alternative therapies exist for treating the above-mentioned pain states including the use of videothoracoscopic splanchnicectomy (VSPL). VSPL has been shown to be almost as effective as neurolytic CPB in patients suffering from chronic pancreatitis pain.27 As pain is commonly identified in pancreatic cancer patients (up to 70% of cases), perhaps the VSPL may be an effective treatment modality employed for that disease as well.28
• For unresectable pancreatic cancer cases, neurolytic CPB reduces opioid consumption and opioid-induced constipation in a majority of patients.29 One study, however, found little difference in opioid consumption between neurolytic CPB, thoracoscopic splanchnicectomy, or patients receiving neither treatment.30
• Clearly, this is an area worthy of additional analysis before final conclusions may be drawn.
• It is also clear that careful selection of appropriate candidates for block, in conjunction with technical factors aimed at enhancing the specificity of blocks, may lead to improved outcomes for pain, increased functionality, and reduction in opioid consumption.31
• A recent National Institutes of Health State-of-the-Science statement on Symptom Management in Cancer highlighted the lack of properly designed comparative analgesic trials for cancer pain, including those consisting of CPB patients.32
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






