Cardioversion and Defibrillation
Naomi F. Botkin
The use of electric countershock to terminate arrhythmia was described over 200 years ago. Thanks to the pioneering work of Zoll et al. [1], Lown et al. [2], and Alexander et al. [3] in the second half of the twentieth century, the use of electric countershock gained widespread acceptance and became a staple in the treatment of cardiac arrhythmia. Cardioversion refers to the use of direct-current electric countershock to terminate arrhythmias other than ventricular fibrillation. In order to avoid triggering ventricular fibrillation, shocks are synchronized with the R wave. Defibrillation, on the other hand, refers to the termination of ventricular fibrillation with unsynchronized shocks. An understanding of both cardioversion and defibrillation is critical for intensive care unit personnel.
Physiology of Arrhythmia and Countershock
Electric countershocks are capable of terminating arrhythmias that are due to reentry. Reentry refers to the phenomenon in which a wave of excitation travels repeatedly over a closed pathway or circuit of conduction tissue. In order for reentry to occur, there must be unidirectional block in one branch of the circuit. In addition, conduction down the unblocked pathway must be slow enough that the blocked pathway recovers excitability by the time the wave reaches the site of block in a retrograde fashion. Continuous electrical activation in such a circuit can lead to reentrant arrhythmias.
Most of the commonly encountered arrhythmias are due to a reentrant mechanism, including atrial fibrillation, atrial flutter, atrioventricular (AV) nodal reentrant tachycardia, most ventricular tachycardias, and ventricular fibrillation. Cardioversion and defibrillation terminate these arrhythmias by simultaneously depolarizing all excitable tissue, disrupting the process of reentry.
Arrhythmias due to disorders all of impulse formation (increased automaticity or triggered activity) do not respond to countershock. These include sinus tachycardia, focal atrial tachycardia, and some types of ventricular tachycardia. Table 6-1 categorizes arrhythmias by their responsiveness to electric countershock.
Insight into the effect of countershock on fibrillating myocardial cells has grown in the past few decades. Although it was initially thought that all ventricular activation fronts had to be terminated simultaneously to stop ventricular fibrillation [4], it is now believed that if the vast majority of myocardium is silenced, the remaining mass is insufficient to perpetuate the arrhythmia [5]. The effect of shock on fibrillating myocardium is complex and is dependent on multiple factors including energy, shock waveform, and myocardial refractory state. It is known that electric countershock at low energy levels may fail to terminate ventricular fibrillation. Although subthreshold shocks may extinguish fibrillatory wavefronts, they will often reinitiate new wavefronts elsewhere in the ventricle, leading to the perpetuation of fibrillation [6]. Thus, it is necessary to deliver shocks above a particular threshold of energy for defibrillation to be successful. Furthermore, ventricular fibrillation can be triggered in patients not already in this rhythm if shocks are poorly timed. Synchronization of shocks with the R wave will minimize the risk.
Indications and Contraindications
Cardioversion and defibrillation are performed for a variety of reasons in the intensive care setting. In the case of hemodynamic instability due to tachyarrhythmia of nearly any variety, the urgent use of countershock is strongly indicated. One must be careful, however, not to mistake sinus tachycardia, which is commonly present in patients who are hypotensive for noncardiac reasons, for a shockable rhythm. The onset of congestive heart failure or angina in a patient with a tachyarrhythmia is also an indication for immediate countershock. In the absence of hemodynamic instability or significant symptoms, cardioversion is usually considered elective and the risks and benefits of the procedure must be carefully weighed.
Extreme caution should be exercised in patients with digitalis toxicity or electrolyte imbalance because of their increased risk of ventricular tachycardia or fibrillation after being shocked. Patients with severe conduction system disease may develop significant bradyarrhythmia after cardioversion. In addition, patients who have been in atrial fibrillation for a prolonged or indeterminate length of time are at risk for thromboembolism due to cardioversion; appropriate measures should be taken to minimize this risk (see below).
Clinical Competence
A clinical competence statement by the American College of Cardiology and American Heart Association outlines the cognitive and technical skills required for the successful and safe performance of elective external cardioversion (Table 6-2). A minimum of eight cardioversions should be supervised before a physician is considered competent to perform the procedure independently. In addition, a minimum of four procedures should be performed annually to maintain competence [7].
TABLE 6-1. Classification of Tachyarrhythmias Based on Predicted Responses to Cardioversion/Defibrillation | |
|---|---|
|
Methods
Patient Preparation
In the case of unconsciousness due to tachyarrhythmia, countershock must be performed urgently. In more elective settings, patient safety and comfort become paramount. As with any procedure, informed consent should be obtained. Patients should refrain from eating and drinking for several hours in order to decrease the risk of aspiration. Constant heart rhythm monitoring should be used throughout the procedure and a 12-lead electrocardiogram should be obtained before and after the countershock.
TABLE 6-2. Cognitive and Technical Skills Necessary for Performing External Cardioversion | ||
|---|---|---|
|
Medications with rapid onset and short half-life are favored for achieving analgesia, sedation, and amnesia. The combination of a benzodiazepine, such as midazolam, and a narcotic, such as fentanyl, is a frequent choice in the absence of anesthesiology assistance. Propofol is often used when an anesthesiologist is present to assist with airway management and sedation. Existing hospital policies for monitoring during conscious sedation should be followed, including frequent assessment of blood pressure and pulse oximetry. Supplemental oxygen is delivered via nasal cannula, face mask, or—in the case of heavier sedation—AmbuBag. The goal of sedation should be minimal or no response to verbal stimulus. In such a state, a patient may cry out during the actual cardioversion but will nonetheless usually have no recollection of the procedure after recovery.
Shock Waveforms
Defibrillators that employ biphasic waveforms have largely replaced those utilizing monophasic waveforms. The chief advantage of biphasic waveforms is a lower defibrillation threshold, meaning shocks using biphasic waveforms require less energy to achieve defibrillation. It is thought that the first phase of the biphasic waveform stimulates the myocardium to defibrillate while the second phase lowers the defibrillation threshold, although the mechanism is not well understood [8]. Biphasic truncated exponential waveform and biphasic rectilinear waveform are both commercially available, with the former being more common. Randomized trials comparing the two types of waveforms in the cardioversion of atrial fibrillation have failed to show any significant difference in efficacy [9, 10 and 11].
The efficacy of biphasic shocks in the termination of VF has been well established [12, 13]. Furthermore, clinical studies of elective cardioversion have established the superiority of biphasic over monophasic waveform shocks [14, 15]. For instance, one study demonstrated the equivalent efficacy of a 120 J to 200 J biphasic sequence with a 200 J to 360 J monophasic sequence [15]. Biphasic waveforms allow fewer shocks to be given and a lower total energy delivery [14]. Whether or not this translates into a significant clinical advantage remains to be demonstrated. However, there is evidence that biphasic shocks result in less dermal injury [14]. Although an animal model suggested better maintenance of cardiac function after biphasic shocks [16], human data on myocardial function are unavailable.
Electrodes
Until recently, hand-held paddles coated with conductive gel were the sole type of electrode used to deliver countershock. Self-adhesive pads have become more common in the past few years, although paddles are still used, especially in emergent cases. Limited data are available comparing the two modalities, but one study suggested the superiority of paddles over pads in cardioverting atrial fibrillation [17]. This phenomenon might be explained by the lower transthoracic impedance achieved with paddles [18]. Whichever modality is used, impedance can be minimized by avoiding positioning over breast tissue, by clipping body hair when it is excessive [19], and by delivering the shock during expiration [20].
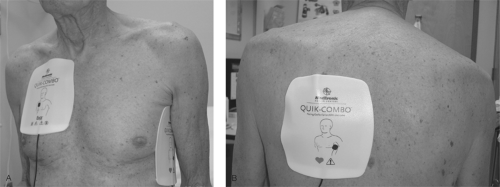
The optimal anatomic placement of pads and paddles is controversial. Anterior-lateral and anterior-posterior placements are both acceptable (Fig. 6-1). The anterior paddle is placed on the right infraclavicular chest [21]. In anterior-lateral placement, the lateral paddle should be located lateral to the left breast and should have a longitudinal orientation, since this results in a lower transthoracic impedance than horizontal orientation [22]. When anterior-posterior positioning is used, the posterior pad is commonly located to the left of the spine at the level of the lower scapula, although some physicians favor placement to the right of, or directly over, the spine. There are data to suggest that anterior-posterior placement is more successful in the cardioversion of atrial fibrillation than anterior-lateral positioning when monophasic waveforms are used [23]. It is thought that anterior-posterior positioning directs more of the delivered energy to the atria than anterior-lateral placement. Since it has been shown that only 4% of the current flow from shock reaches the myocardium with the anterior-lateral position [24], any method that directs more energy to the atria should be beneficial. However, a study using defibrillators employing a biphasic waveform suggested pad position was not associated with cardioversion success [25].
TABLE 6-3. Checklist for Performing Cardioversion
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|
|---|