INTRODUCTION
The term cardiomyopathy describes a heterogeneous group of diseases that directly alter cardiac structure, impair myocardial function, or alter myocardial electrical properties. Discoveries in molecular genetics and the description of (ion) channelopathies as diseases have prompted new definitions and classification of cardiomyopathies.1 Primary cardiomyopathies are diseases that solely or predominantly involve the myocardium1; the most common disorders are listed in Table 55-1. Secondary cardiomyopathies include heart muscle diseases associated with specific systemic disorders. At present, no classification method perfectly distinguishes all forms of cardiomyopathy, and overlap exists between categories.2,3 Secondary cardiomyopathies often present with hemodynamic findings similar to those of the idiopathic dilated or restrictive forms of cardiomyopathy. The most common causes of secondary cardiomyopathies are listed in Table 55-2. As a group, the cardiomyopathies are the third most common form of cardiac disease encountered in the United States, following coronary (ischemic) heart disease and hypertensive heart disease. Hypertrophic cardiomyopathy is the second most common cause of sudden cardiac death in the adolescent population and the leading cause of sudden death in competitive athletes.4
Genetic
Mixed (genetic and nongenetic)
Acquired
|
Toxins
Infiltrative diseases
Storage diseases
Autoimmune disorders
Metabolic
Neuromuscular disorders
|
An in-depth discussion of each of the primary and secondary cardiomyopathies is beyond the scope of this chapter, and one is unlikely to make a specific diagnosis in the ED. This chapter discusses selected cardiomyopathies (Table 55-3). The cardiomyopathies usually present with signs of systolic and diastolic ventricular dysfunction. The ED evaluation will generally guide the need for urgent treatment, admission, or referral for further diagnostic evaluation, based on the severity of symptoms.
| Type | Name | Clinical Features | ECG |
|---|---|---|---|
| Systolic and diastolic dysfunction | Dilated cardiomyopathy | Congestive heart failure Chest pain Regurgitant murmurs | LVH Poor R-wave progression |
| Myocarditis | Fever Tachycardia Myalgias Chest pain | Nonspecific ST-T wave changes, often with pericarditis | |
| Diastolic dysfunction | Hypertrophic cardiomyopathy | Dyspnea on exertion Chest pain Palpitations Syncope Prominent J wave Pulsus bisferiens Systolic ejection murmur, increases with Valsalva and decreases with squatting | LVH Large septal Q waves |
| Restrictive cardiomyopathies | “Square root sign” of left ventricular filling pressures; easily confused with constrictive pericarditis | In some, low voltage of QRS; conduction disturbances; atrial fibrillation |
CARDIOMYOPATHIES WITH SYSTOLIC AND DIASTOLIC DYSFUNCTION
Dilated cardiomyopathy is the most common cardiomyopathy and is usually idiopathic but may be familial or occur with specific cardiac or systemic disorders (Tables 55-1 and 53-2). Peripartum cardiomyopathy most commonly manifests as dilated cardiomyopathy and is discussed in chapter 100, “Maternal Emergencies after 20 Weeks of Pregnancy and in the Postpartum Period.” The idiopathic form of dilated cardiomyopathy causes approximately 25% of all cases of congestive heart failure (CHF) and is the primary indication for cardiac transplantation in the United States. The prevalence of idiopathic dilated cardiomyopathy is about 36 cases per 100,000 population. Blacks and males have a 2.5-fold increase in risk compared with whites and females. Most patients are diagnosed between the ages of 20 and 50 years, and the majority have advanced symptoms of CHF at the time of initial presentation.5
Dilated cardiomyopathy is characterized by systolic and diastolic dysfunction and diminished left ventricular (LV) and, often, right ventricular (RV) contractile force, resulting in a low cardiac output and increased end-systolic and end-diastolic ventricular volumes. A decrease in ventricular compliance leads to an increase in intracavitary pressures. LV and, often, RV dilatation accompanied by normal LV wall thickness are the hallmarks of dilated cardiomyopathy.
As a result of systolic pump failure, the patient presents with signs and symptoms of CHF: dyspnea on exertion, orthopnea, paroxysmal nocturnal dyspnea, bibasilar rales, and dependent edema. Depressed ventricular contractile function and dilatation may result in the formation of mural thrombi, and the patient may develop signs of peripheral embolization (e.g., an acute neurologic deficit, flank pain, and hematuria or a pulseless, cyanotic extremity). Chest pain is felt to be due to limited coronary vascular reserve rather than atherosclerotic coronary artery disease, but the cause cannot be distinguished clinically.
Murmurs do not necessarily indicate primary valvular disease. Annular dilatation and displacement of the papillary muscles of the atrioventricular valves inhibit complete valve closure. Holosystolic mitral or tricuspid regurgitant murmurs are frequently heard at the apex or lower left sternal border. An apical diastolic rumble may be heard and is due either to accentuated, early diastolic atrial-to-ventricular flow (the result of mitral regurgitation and left atrial overload) or to a loud summation gallop. The liver will be enlarged and pulsatile if tricuspid insufficiency is significant.
The diagnosis may be suspected in patients with typical radiographic and electrocardiographic findings, but the diagnosis is typically made at follow-up with echocardiography and additional testing as indicated by individual characteristics. The chest radiograph invariably shows an enlarged cardiac silhouette and increased cardiothoracic ratio. Biventricular enlargement is common. Evidence of pulmonary venous hypertension (“cephalization” of flow and enlarged hila) is also frequent and may serve to differentiate cardiac enlargement due to myocardial failure from that due to a large pericardial effusion.
The electrocardiogram is almost always abnormal. LV hypertrophy and left atrial enlargement are the most common findings. Q or QS waves and poor R-wave progression across the anterior precordium may produce a pseudoinfarction pattern. Atrial fibrillation and ventricular ectopy are common rhythm disturbances.
Echocardiographic studies in a symptomatic patient demonstrate a decreased ejection fraction, increased systolic and diastolic volumes, and ventricular and atrial enlargement.6 Echocardiography is indicated when the cause of heart failure is uncertain to exclude known causes of heart failure that may be correctable (e.g., pericardial effusion or valvular disease), to estimate ejection fraction, and to rule out other potential complications (e.g., mural thrombi) that may be amenable to therapy. The acuity of the patient’s presentation determines the urgency of echocardiography.
For the treatment of acute decompensated heart failure, see chapter 53, “Acute Heart Failure.” Chronic therapy may include diuretics and digoxin, but these drugs do not appear to improve survival rates.7 Guidelines from the American College of Cardiology for the management of heart failure (and other cardiovascular disorders) are available online at http://www.cardiosource.org/Science-And-Quality/Practice-Guidelines-and-Quality-Standards.aspx. The use of angiotensin-converting enzyme inhibitors and blockers, specifically carvedilol, improves survival in patients with dilated cardiomyopathy and CHF.8 Selected patients benefit from cardiac resynchronization therapy.9,10,11 Patients with complex ventricular ectopy who are found to be at risk for sudden cardiac death may benefit from amiodarone therapy or an implanted cardioverter-defibrillator.12
Patients with a known dilated cardiomyopathy and chronic CHF may present to the ED with a mild to moderate worsening of symptoms.13 If the cause is noncompliance with medical therapy or diet, ED treatment with nitrates, IV diuretics, reinstitution of prescribed medications, patient counseling, and timely referral to the primary care physician are appropriate. However, life-threatening causes of acute exacerbations, such as cardiac ischemia, should be considered before assuming benign causes. Acutely symptomatic patients require hospitalization for definitive diagnosis and management. Important subsets of patients with dilated cardiomyopathy are treated with left ventricular assist devices while awaiting heart transplantation or as destination therapy.14,15 Principles of patient assessment and management and device complications are described in Left Ventricular Assist Devices (see next page).
Myocarditis is a common cause of dilated cardiomyopathy but is discussed separately to highlight its acute presentation and individual therapy. Myocarditis is inflammation of the heart muscle and is most frequently characterized pathologically by focal infiltration of the myocardium by lymphocytes, plasma cells, and histiocytes. Varying amounts of myocytolysis and destruction of the interstitial reticulin network are also seen.16 Because many episodes are mild, they do not always come to medical attention. Table 55-4 lists some common infectious causes of myocarditis. Myocarditis is frequently accompanied by pericarditis.
| Viral Agents | Bacteria |
|---|---|
| Coxsackie B virus | Corynebacterium diphtheriae |
| Echovirus | Neisseria meningitidis |
| Influenza virus | Mycoplasma pneumoniae |
| Parainfluenza virus | β-Hemolytic streptococci (rheumatic fever) |
| Epstein-Barr virus | Lyme disease |
| Hepatitis B virus | |
| Human immunodeficiency virus |
LEFT VENTRICULAR ASSIST DEVICES (LVADs)
Daniel Renner, Heather Heaton, and Jason N. Katz
Left ventricular assist devices (LVADs) augment left ventricular output in patients with severe cardiomyopathy. Right ventricular assist devices and biventricular assist devices also exist, though these are much less common and are used only for temporary circulatory support. There are several models of LVADs in current use which share many similar features.
In all LVADs, the implanted pump transfers blood from the apex of the left ventricle to the proximal aorta (Figure 1). The pump is powered by an external power source (battery or bedside unit), which is connected to a controller. Both the battery and controller reside outside of the body and can be carried or worn by the patient. The controller drives the pump through a driveline, which connects the implanted pump to the external controller through a surgical incision in the abdominal wall.
Most contemporary LVADs use pumps that drive blood through a continuous-flow mechanism (e.g. axial or centrifugal flow), maintaining a normal mean arterial blood pressure in the absence of a palpable pulse. However, many patients may still retain some cardiac contractility; in these individuals the LVAD serves to assist (and not replace) normal physiologic cardiac output, and a pulse may be present. Because the LVAD can (in some patients) maintain systemic perfusion even with minimal cardiac function, an LVAD patient can sometimes be clinically stable even in the setting of ventricular fibrillation. LVAD patients still rely upon reasonable right ventricular function to pump blood to the lungs.
Patients and families are thoroughly trained on the management of the LVAD and its complications, and they should be involved during patient assessment and management. It is important to call the patient’s LVAD coordinator as soon as possible to assist with management decisions–this information should be present in the patient’s travel bag, along with a spare controller and batteries.
CLINICAL FEATURESLVAD patients should have a normal mean arterial pressure. Blood pressure can be assessed by a mechanical cuff or by doppler ultrasound. If using a doppler, remember that blood flow can be continuous and not pulsatile. When auscultating the heart, the continuous whirr of the pump is heard.
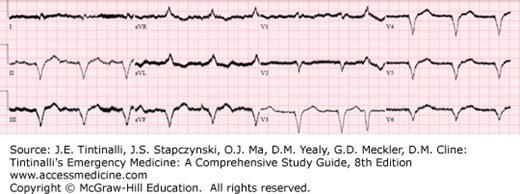
The ECG should have discernible QRS complexes (Figure 2).
The LVAD is visible on a chest radiograph (Figure 3). Bedside US can be used to assess RV function and to evaluate for pericardial effusion/tamponade. Plain imaging and CT scans are safe, but MRIs are contraindicated.
THE HEMODYNAMICALLY UNSTABLE LVAD PATIENTNEVER PERFORM CHEST COMPRESSIONS ON AN LVAD PATIENT. Chest compressions can dislodge the LVAD from the heart and aorta, causing LV rupture and intractable hemorrhage.
Immediately auscultate the precordium – an audible “whirr” indicates that the pump is functioning. If nothing is heard, search for a cause of mechanical LVAD failure. With the help of family or the patient, check and/or change the batteries and/or controller. Do not disconnect anything.
If the pump is audible and functioning, obtain a blood pressure (by automatic cuff or manual doppler) and place the patient on a cardiac monitor and continuous pulse oximetry. If the LVAD model does not provide pulsatile flow, it is difficult to obtain a reading by pulse oximetry.
For hypotension, give a bolus of normal saline. Assess for bleeding, especially from GI hemorrhage. If hypotension persists despite adequate fluid resuscitation, or in the presence of right ventricular failure or LVAD malfunction, initiate IV pressors. Dopamine is a reasonable first line therapy. Obtain an ECG to rule out right ventricular myocardial infarction or strain. Obtain standard laboratory studies as clinically indicated. Bedside US can assess for right ventricular dilatation or failure. If there is right ventricular strain, consider pulmonary hypertension or pulmonary embolism. Give heparin if pulmonary embolism or device thrombosis is suspected and bleeding is excluded.
VF or VT in an unstable patient requires defibrillation/cardioversion, using standard ACLS energy recommendations. Do not place defibrillator pads over the driveline. If the patient is clinically stable, give amiodarone according to ACLS protocols.
MEDICAL COMPLICATIONSMedical complications include anemia, bleeding, thromboembolism, or infection. Anemia can be caused by hemolysis (erythrocyte destruction from the pump) or bleeding. LVAD patients are typically anticoagulated with warfarin to an INR target of 2-3. Bleeding and coagulopathy are investigated and treated with standard measures. LVAD patients are also at risk for thromboembolism, such as pulmonary embolism, stroke and mesenteric ischemia, especially in the setting of suboptimal anticoagulation. Heparin is safe and indicated for such events once bleeding has been ruled out. Infection is a common complication, especially at the driveline exit site. Treat sepsis with volume resuscitation, blood cultures, and antibiotics.
Fever, myalgias, headache, and sinus tachycardia usually out of proportion with fever are common signs and symptoms. Other signs and symptoms depend on the extent of myocardial involvement and myocardial depression. In severe cases, heart failure can develop. With less extensive myocardial involvement, pericarditis and the clinical manifestations of systemic illness (fever, myalgias, headache, and rigors) may overshadow clinical signs of myocardial dysfunction, and myocarditis may not be suspected. Retrosternal or precordial angina-type chest pain is frequent and is usually due to pericardial inflammation (myopericarditis). A pericardial friction rub is commonly heard.
The gold standard for diagnosis of myocarditis is endocardial biopsy, but this invasive modality is uncommonly used.17 The diagnosis is more commonly made clinically based on symptoms with supportive testing. The chest radiograph is usually normal or nondiagnostic. Cardiomegaly and pulmonary venous hypertension or pulmonary edema are present with severe disease. Electrocardiogram changes include nonspecific ST-T–wave changes, ST-segment elevation or PR depression from associated pericarditis, atrioventricular block, and QRS interval prolongation. Cardiac enzymes may be elevated.18 Echocardiographic studies are also nonspecific, with myocardial depression and wall motion abnormalities in severe cases. Newer imaging modalities include nuclear imaging with gallium-67– or indium-111–labeled antimyosin antibodies and cardiac MRI.19,20,21
Treatment for idiopathic or viral myocarditis is supportive. Antibiotics are needed for myocarditis complicating rheumatic fever, diphtheria, or meningococcemia. Immunosuppressive therapy (e.g., prednisone, azathioprine, and others) may be of value in selected patients, but large trials have not consistently demonstrated benefit.19 Immunosuppressive therapy is usually reserved for more severe cases and is rarely begun in the ED. Admission is usually indicated if the patient presents with CHF.