INTRODUCTION AND EPIDEMIOLOGY
Cardiogenic shock is an acute state of decreased cardiac output resulting in inadequate tissue perfusion despite adequate circulating volume. Cardiogenic shock is the leading cause of in-hospital death in patients with acute myocardial infarction (AMI).1 The true incidence of cardiogenic shock is unknown because many patients die before arrival and escape estimates. Cardiogenic shock is seen in 4% to 8% of patients with ST-segment elevation myocardial infarction (STEMI).2,3 The incidence is declining in part as a result of the increased use of percutaneous intervention for AMI.3,4,5,6 Cardiogenic shock occurs less frequently (2.5%) in those with non–ST-segment elevation myocardial infarction (NSTEMI) compared with those with STEMI.7,8 Only ~10% of AMI patients who will develop cardiogenic shock have it at ED presentation, with the median time of onset after arrival being approximately 6 hours.2,9 This underscores the therapeutic opportunity that exists by thwarting ongoing myocardial ischemia.
During the past decade, a strategy of early revascularization by percutaneous coronary intervention or coronary artery bypass surgery improved survival of cardiogenic shock patients with acute ischemia compared to medical therapy alone.3,10,11,12 Despite these advances, the mortality remains high (~50%), with half of the deaths occurring within the first 48 hours after presentation.3,13,14,15 Early recognition of cardiogenic shock or ongoing myocardial ischemia is the key for emergency physicians. Prompt and successful efforts to restore perfusion optimize patient outcomes.
The more risk factors that are present (Table 50-1), the greater is the amount of vulnerable myocardium and the greater is the likelihood of cardiogenic shock.
Elderly Female Acute or prior ischemic event associated with the following: Impaired ejection fraction Extensive infarct (evidence of large myocellular leak) Proximal left anterior descending coronary artery occlusion Anterior myocardial infarction Multivessel coronary artery disease Prior medical history: Previous myocardial infarction Congestive heart failure Diabetes |
PATHOPHYSIOLOGY
The most common cause of cardiogenic shock is extensive myocardial infarction that depresses myocardial contractility. Additional causes are listed in Table 50-2. Regardless of the precipitating cause, cardiogenic shock is primarily “pump failure,” which results in reduced cardiac output. The systolic blood pressure drops due to poor cardiac output, and vital organ perfusion is limited. Absent a rise in systemic vascular resistance, the diastolic blood pressure also drops, resulting in coronary artery hypoperfusion. This creates a cycle of worsening myocardial ischemia and pump dysfunction, and eventual decompensation.
Mechanical complications: Acute mitral regurgitation secondary to papillary muscle dysfunction or chordal rupture Ventricular septal defect Free wall rupture Right ventricular infarction Acute aortic insufficiency (aortic dissection) Severe depression of cardiac contractility: Acute myocardial infarction Sepsis Myocarditis Myocardial contusion Cardiomyopathy Medication toxicity (e.g., β-blocker overdose, calcium channel blocker overdose) Unstable dysrhythmia Mechanical obstruction to forward blood flow: Aortic stenosis Hypertrophic cardiomyopathy Mitral stenosis Left atrial myxoma Pericardial tamponade |
Historically, many believed cardiogenic shock was associated with a reflex compensatory vasoconstriction that would increase systemic vascular resistance. Contemporary data refute this belief, showing the average systemic vascular resistance was not elevated in cardiogenic shock patients, even with vasopressor use.16 Furthermore, the average left ventricular ejection fraction (EF) was only moderately depressed (~30%),17 and diastolic dysfunction was seen early and frequently, and was associated with greater depression of EF and an increased need for mechanical support.18,19
A systemic inflammatory response syndrome occurs after AMI and in cardiogenic shock, due to complement system activation and release of systemic inflammatory mediators, including cytokines and inducible nitric oxide synthase.20,21 The inflammatory response also depresses pump function, dilates the peripheral vasculature, and increases the risk of death.22 However, targeting nitric oxide with tilarginine does not alter mortality, although it improves blood pressure.23 The monoclonal c5 antibody pexelizumab has been studied as an adjunct to percutaneous intervention in an effort to blunt the activation of the complement cascade associated with infarction. The largest trial of pexelizumab failed to show a mortality benefit compared to placebo.24
Resolution of severe ischemia, neurohormonal, and inflammatory abnormalities may explain the reversible nature of cardiogenic shock in some patients. The wide variations in EF, ventricular size, and vascular resistance suggest that the pathophysiology of cardiogenic shock is diverse and poorly understood.
CLINICAL FEATURES
History can be difficult to obtain if the patient is severely ill. EMS personnel, family, or the medical record may offer additional historical information, notably of existing ischemic heart disease. Patients commonly complain of shortness of breath, chest pain, or weakness. Through history, try to exclude other causes of shock, such as sepsis, massive pulmonary embolism, hemorrhage, or a viral prodrome suggesting myocarditis. Ask about a history of preexisting valvular disease, recent illnesses, hypercoagulable states, substance abuse, or other risk factors for cardiogenic shock as outlined in Table 50-1. Assess for other causes of shock because treatment differs depending on the cause of cardiovascular system failure (Table 50-3).
Acute pulmonary decompensation: Chronic obstructive pulmonary disease exacerbation Cor pulmonale Massive pulmonary embolism Distributive shock: Sepsis Anaphylaxis Neurogenic shock (spinal cord injury) Hypovolemic shock: Hemorrhage Severe dehydration Dissociative shock: Toxins/drugs of abuse (cyanide) |
Cardiogenic shock is characterized by hypoperfusion; this is not always accompanied by hypotension.9 Systolic blood pressure is usually <90 mm Hg, although it can be higher with preexisting hypertension. A pulse pressure <20 mm Hg is another finding if systemic resistance has not plummeted, and sinus tachycardia is common unless the patient is on medications that block a tachycardic response. Unless the patient has advanced to the stage of respiratory fatigue or agonal respirations, tachypnea is common. The lung examination demonstrates rales due to the presence of pulmonary edema, except in cases of isolated right-sided failure. Jugular venous distention and a positive hepatojugular reflex are usually present. Patients are usually pale or cyanotic and may have cool skin and mottled extremities or other signs of hypoperfusion. Peripheral edema suggests preexisting heart failure. Diaphoresis indicates activation of the sympathetic nervous system. Cerebral hypoperfusion may result in altered mental status, and renal hypoperfusion may decrease urine output.
If the cardiac point of maximal impulse is normally located, shock is likely due to an acute event. If the point of maximal impulse is laterally shifted and diffuse from cardiac remodeling and enlargement, long-standing cardiac disease with acute decompensation can be presumed. About 10% of cardiogenic shock after AMI is caused by mechanical complications.14 A new murmur may be the only physical exam finding of mechanical catastrophe; carefully seek any loud or new systolic murmurs. Acute mitral regurgitation can occur from chordae tendineae rupture or papillary muscle dysfunction, accompanied by a soft holosystolic murmur at the apex radiating to the axilla with rales. With papillary muscle dysfunction, the murmur starts with the first heart sound but terminates before the second. An acute ventral septal defect is associated with a new loud holosystolic left parasternal murmur, often with a palpable thrill, that decreases in intensity as the intraventricular pressures equalize. Acute aortic insufficiency is characterized by a soft diastolic murmur and a softer S1 sound.
DIAGNOSIS
Clinical signs of cardiogenic shock include evidence of poor cardiac output with tissue hypoperfusion (hypotension, mental status changes, cool mottled skin) and evidence of volume overload (dyspnea, rales, jugular venous distention). Hemodynamic criteria for cardiogenic shock include (1) sustained hypotension (systolic blood pressure <90 mm Hg), (2) reduced cardiac index (<2.2 L/min/m2), and (3) an elevated (>18 mm Hg) pulmonary artery occlusion pressure. The causes and differential diagnosis of cardiogenic shock are listed in Tables 50-2 and 50-3.
There are no laboratory markers specific for the diagnosis of cardiogenic shock. Cardiac biomarkers (primarily troponin) may not be elevated upon initial presentation from an acute myocardial ischemic triggering event, but will eventually elevate. A CBC excludes anemia, which can contribute to cardiac ischemia. The clinical presentation guides the need for specific drug levels (e.g., digoxin, ethanol, or illicit drugs). Hypoperfusion commonly results in an elevated serum lactate, so checking serum lactate may aid diagnosis when overt hypotension is absent. Serum electrolytes and renal and hepatic studies can identify end-organ dysfunction.
The level of serum B-type natriuretic peptide (BNP) is an indicator of left ventricular dysfunction. Because of its high negative predictive value, a normal BNP level (<100 picograms/mL) eliminates cardiogenic shock as the cause of hypoperfusion unless very early after onset or with isolated right heart failure. Conversely, an elevated BNP does not diagnose cardiogenic shock.25,26
Although elevated inflammatory markers such as C-reactive protein have some prognostic value, these are rarely needed in the acute phase of care.27 Arterial blood gas measurements help identify those at risk of carbon dioxide retention, quantify the presence and severity of acidosis, and determine the contribution of metabolic or respiratory components to acidosis.
The ECG helps detect ischemia or STEMI, evaluates for rhythm abnormalities, and provides evidence of electrolytic abnormalities (e.g., hypokalemia) or drug toxicity (e.g., digoxin).
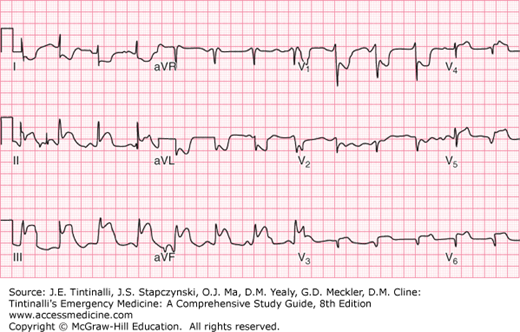
It is important to assess for right ventricle (RV) involvement whenever ischemia is considered because RV infarction is associated with an increased risk for cardiogenic shock and death.28 RV infarction is best evaluated by obtaining right-sided ECG leads (usually V4R and V5R) (Figure 50-1). RV infarction complicating inferior myocardial infarction is detected by ST elevation in lead V1 with depression in V2.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree