Chapter 88
Cardiac Surgery
Effects of Cardiopulmonary Bypass
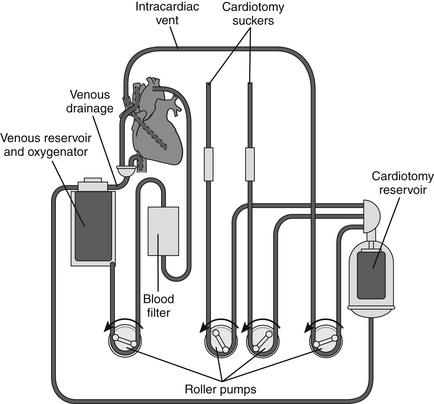
The basic CPB circuit (Figure 88.1) is composed of a large venous catheter (inflow), a reservoir, a centrifugal or roller pump, an oxygenator, a heat exchanger, a debubbler, and an arterial catheter (outflow). Inflow of venous blood to the reservoir occurs primarily by gravity. Depending on the procedure, these venous catheters can be placed either centrally (e.g., right atrium, inferior vena cava, superior vena cava) or peripherally (e.g., femoral vein, internal jugular vein). Blood loss in the operative field is collected by pump suckers and also returned to the venous reservoir. Reservoir blood is then pumped through the oxygenator and heat exchanger. In turn, the warmed and oxygenated blood is infused into the arterial system via a cannula placed either centrally (e.g., ascending aorta, aortic arch) or peripherally (e.g., femoral or axillary artery).
Complement activation induced by exposure of plasma to the foreign CPB circuit causes a generalized inflammatory reaction characterized by the release of multiple cytokines, lymphokines, and proteases. Furthermore, this cascade of events augments platelet activation, coagulation, and fibrinolysis. The resulting coagulation contributes to ischemia-reperfusion injury in various end organs. Platelet disruption occurs as a result of direct contact with the circuit’s inner surface, especially at the level of the oxygenator. Platelet dysfunction also occurs as a consequence of complement-induced opsonization in addition to the generalized inflammatory state. Ultimately, the fibrinolytic pathway is activated by CPB increasing levels of tissue plasminogen activator (tPA) in response to endothelial damage, platelet activation, and complement activation. Hemodilution and consumption of these clotting factors contribute to a bleeding diathesis postoperatively. Red blood cell disruption, which manifests as hemoglobinuria, correlates directly with the intensity of pump sucker use as well as with the total duration of CPB. White blood cell activation and subsequent sequestration in the lungs can occur as a result of CPB. When combined with complement activation and high levels of circulating cytokines, this can lead to pulmonary edema and injury and, rarely, postoperative acute respiratory distress syndrome (ARDS).
Evaluation of Cardiac Function in the Intensive Care Unit
Invasive Hemodynamic Monitoring
Various forms of invasive hemodynamic monitoring are used to guide postoperative recovery of heart function, fluid management, and overall management in the ICU setting (Chapter 7). Continuous blood pressure is monitored via a radial or femoral arterial catheter. Many patients also have a pulmonary arterial catheter (PAC) in place, which can be helpful in assessing both right and left heart function. Importantly, in patients with a PAC, great caution should be taken if a pulmonary artery wedge pressure (PAWP) is deemed necessary, as many of these patients have multiple factors, including pulmonary hypertension and an acquired bleeding diathesis, that put them at high risk for balloon-induced rupture of the pulmonary artery, a usually fatal complication.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree