CARDIAC EMERGENCIES
CASANDRA QUIÑONES, MD AND BETH BUBOLZ, MD
GOALS OF EMERGENCY THERAPY
Pediatric cardiac emergencies are caused by a wide variety of pathophysiologic states and so have a variety of presentations. Cardiac emergencies may be due to congenital heart disease (CHD), arrhythmias, acute heart failure syndromes (AHFS), trauma, and sequelae of treatment. The common denominator in cardiac emergencies is that cardiac output is compromised. The challenge for emergency medicine (EM) providers is to identify cardiac emergencies promptly even when the chief complaint is not cardiac in nature. With the proper mental model for interpreting the patient’s signs and symptoms, the provider can determine the correct approach to these complex patients. The clinician must consider heart disease when evaluating common symptoms such as feeding difficulty, abdominal pain, wheezing, or respiratory distress and can make the correct diagnosis if the proper framework for interpreting signs and symptoms is applied.
If the provider understands framework, an exhaustive knowledge of every anatomic variation of CHD is not necessary. Instead, the EM provider can recognize cardiac disease by the presenting symptoms and the patient may be assessed rapidly and accurately and restoration of adequate cardiac output can be started.
KEY POINTS
 CHD should be a consideration in any neonate presenting with acute decompensation in the first 2 months of life.
CHD should be a consideration in any neonate presenting with acute decompensation in the first 2 months of life.
 CHD often presents with cyanosis or shock in the first 2 weeks of life, coinciding with closure of the ductus arteriosus (DA).
CHD often presents with cyanosis or shock in the first 2 weeks of life, coinciding with closure of the ductus arteriosus (DA).
 CHD often presents with pulmonary over circulation and poor feeding around 2 months of life coinciding with fall in pulmonary vascular resistance (PVR).
CHD often presents with pulmonary over circulation and poor feeding around 2 months of life coinciding with fall in pulmonary vascular resistance (PVR).
 Pediatric patients with AHFS often present with nonspecific, noncardiac complaints many times before the correct diagnosis is made.
Pediatric patients with AHFS often present with nonspecific, noncardiac complaints many times before the correct diagnosis is made.
 Incessant tachycardia may lead to heart failure at any age.
Incessant tachycardia may lead to heart failure at any age.
 Children with implanted cardiac devices may present with complications of implantation or device failure.
Children with implanted cardiac devices may present with complications of implantation or device failure.
RELATED CHAPTERS
Resuscitation and Stabilization
• Cardiopulmonary Resuscitation: Chapter 4
Medical Emergencies
• Neonatal Emergencies: Chapter 104
Signs and Symptoms
• Dizziness and Vertigo: Chapter 19
• Rash: Bacterial and Fungal Infections: Chapter 61
• Respiratory Distress: Chapter 66
• Septic Appearing Infant: Chapter 68
CONGENITAL HEART DISEASE
Goals of Treatment
The goals of treatment are the rapid recognition, stabilization, and restoration of cardiac output in patients who present with undiagnosed congenital or acquired heart disease as well as the stabilization of a child with an underlying heart condition who presents with an emergent noncardiac condition.
CLINICAL PEARLS AND PITFALL
• Recognition of the most common presentation patterns for patients with CHD will guide appropriate therapy.
• CHD should be a consideration in any neonate presenting with acute decompensation.
• Evaluate femoral pulse in all newborns and infants to detect coarctation of the aorta.
• Palliation or incomplete repair of a congenital heart defect in an infant is a red flag for a patient who may not tolerate other stressors.
• Pediatric patients with cardiac disease often present with nonspecific symptoms involving the gastrointestinal or pulmonary systems.
• Endotracheal intubation and mechanical ventilation can significantly decrease cardiac demands.
Current Evidence
Congenital heart malformations are the most common types of birth defects, affecting nearly 1% or approximately 40,000 live births per year in the United States. Nearly half of the deaths from CHD occur in the first year of life. Recent data reveals that 15% of CHD is associated with chromosomal abnormalities. Twenty-nine percent of CHD is associated with other major noncardiac malformations. This is a complex patient population who often seek medical care in the ED.
TABLE 94.1
GLOSSARY OF CONGENITAL HEART DEFECTS
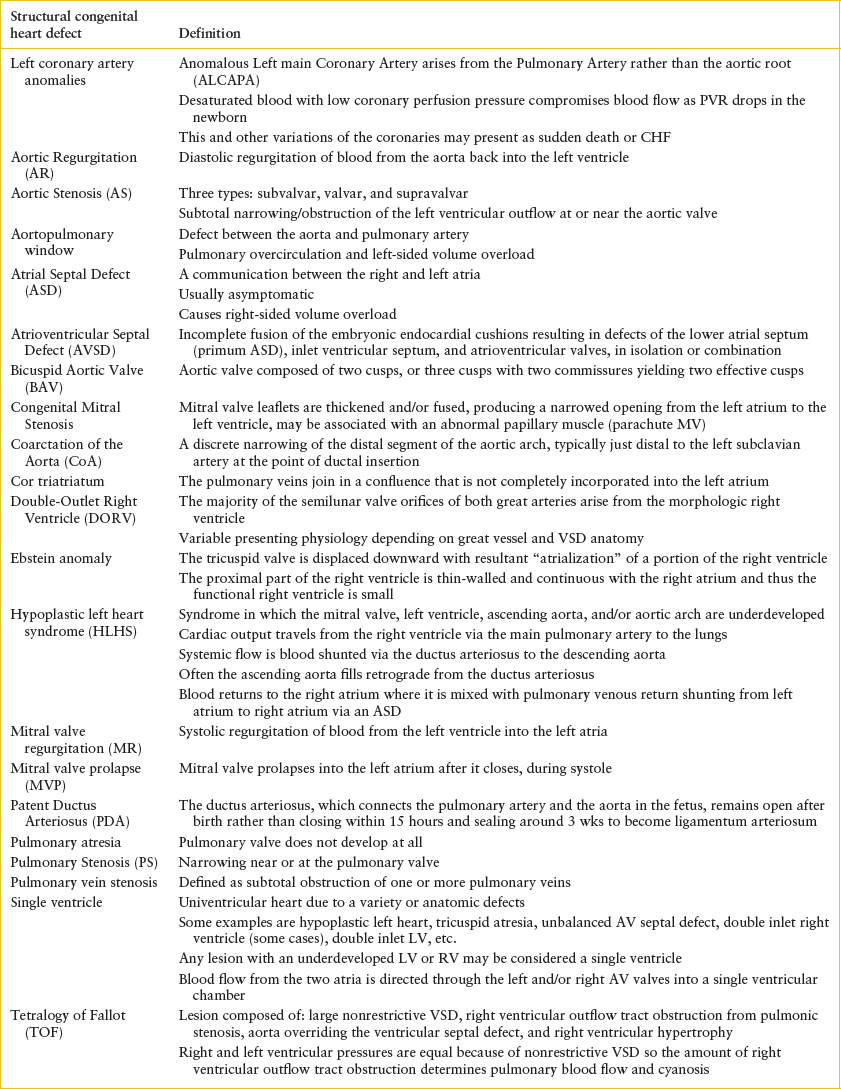
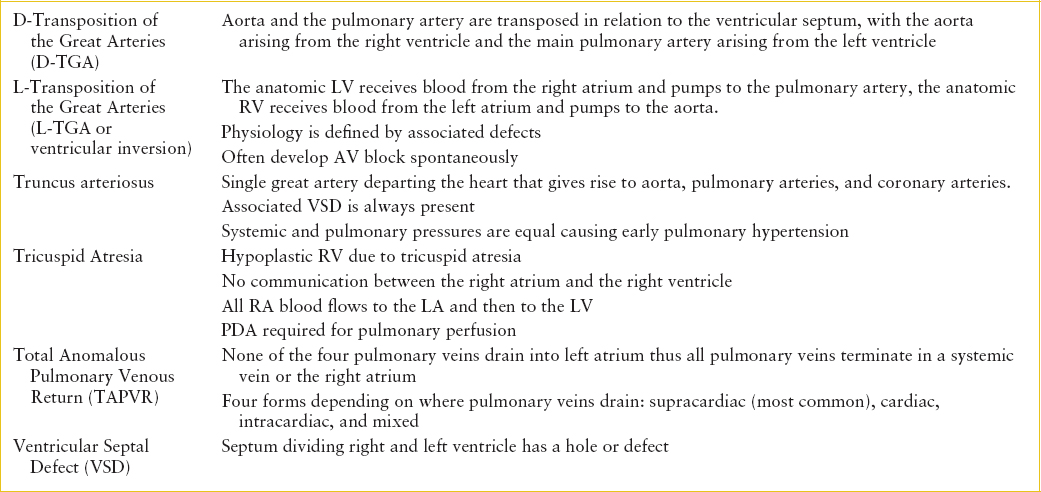
All forms of CHD occur along a spectrum. For example, tetralogy of Fallot (TOF) may range from pulmonary atresia to very mild pulmonary stenosis, or absent pulmonary valve (no stenosis). Therefore, presentation of TOF can range from fulminant cyanosis and shock to mild cyanosis to congestive heart failure (CHF). It is not necessary to know the exact anatomical defect at the time of presentation but rather focus on the cardiac physiology. Anatomical details will be defined by an echocardiogram, but that full information is not necessary for recognition of common patterns of presentation and stabilization in the emergency department (ED).
Clinical Considerations
Clinical Recognition
Many times when an infant or child presents to the ED, the diagnosis is not immediately evident and management is initiated without complete certainty of the underlying pathology. This is especially true with CHD. However, a framework can be used to assist in forming a differential diagnosis, instituting therapy, and communicating with the pediatric cardiologist. This approach encourages the provider to categorize patients into groups according to their presenting vital signs and predominant symptoms.
Presentation of CHD often occurs at the time of transition from fetal to postnatal circulation. The adaptation to extra-uterine life involves closure of three shunts: The DA, patent foramen ovale in the atrial septum, and ductus venosus. Pulmonary vascular resistance (PVR) drops dramatically with the first breath and continues to diminish significantly over the next 2 months. It follows that closure of the DA and fall in PVR may cause infants with cardiac malformations to become symptomatic.
Structural CHD can be divided into four major categories. Each of the four categories has typical presenting symptoms due to the underlying hemodynamics. The key to diagnosis is associating presenting symptoms and physical findings with each of the major categories of CHD. The four categories of structural CHD are: (1) Ductal dependent lesions that require a patent ductus arteriosus (PDA) for pulmonary blood flow (PBF); (2) ductal dependent lesions that require a PDA for systemic blood flow; (3) shunt lesions with right-to-left flow; and (4) shunt lesions with left-to-right flow (Tables 94.1 and 94.2).
Categorization of a patient into one of these four groups requires knowing the patient’s age, weight, vital signs, and physical examination findings, which all may be gathered quickly. This simplified method of understanding heart disease can help the provider identify and treat emergencies due to CHD (Table 94.3 and  e-Table 94.1).
e-Table 94.1).
Ductal Dependent Lesions
After birth, circulating prostaglandins decrease and the DA naturally closes. Cardiac defects, which depend on ductal patency for stable circulation, will present at this time, typically within the first 2 weeks of life. Either pulmonary or systemic blood flow may be dependent on a PDA. These lesions have very different presentations, which can be recognized by the astute clinician.
Ductal Dependent Pulmonary Blood Flow
Cardiac defects in which the pulmonary outflow tract is atretic depend completely on patency of the DA for survival. When the ductus closes, these patients become extremely hypoxic and present with severe cyanosis and shock. Defects such as critical pulmonary stenosis, pulmonary atresia with intact ventricular septum, and severe TOF, present in this dramatic fashion.
Less severe forms of ductal dependency for PBF present with varying degrees of cyanosis at the time this vessel closes. The degree of cyanosis is determined by the amount of blood flow to the lungs. These infants feed and grow well initially, have normal respiratory rate and effort, and normal vital signs. Defects such as TOF and some forms of tricuspid atresia and double outlet right ventricle (DORV) present in this fashion.
Ductal Dependent Systemic Blood Flow
Patients with severe left ventricular outflow tract obstructive (LVOTO) lesions depend on blood flow from the pulmonary artery via the DA to supply the descending aorta. By definition, this is a right-to-left shunt that causes desaturated blood to be circulated systemically, and it is necessary to provide systemic blood flow. Cyanosis is usually mild. Early symptoms include mild tachypnea or cyanosis, which can quickly progress to cardiogenic shock when the ductus closes. Cardiovascular collapse may be the first indication that there is a heart defect. Resulting acidosis and pressure loading of the ventricle accelerate decompensation and end-organ failure rapidly follows. LVOTO lesions such as hypoplastic left heart syndrome (HLHS), critical aortic stenosis, and severe coarctation of the aorta present in this fashion.
Shunt Lesions
Left-to-right Shunts
Left-to-right shunt lesions allow oxygenated blood to pass from the systemic circulation to the pulmonary circulation. The amount of flow is determined by the size of the defect and the relative PVR compared to systemic vascular resistance (SVR). If the PVR is high or if the defect is small, left-to-right shunting is limited and the patient is relatively asymptomatic. As PVR drops, left-to-right shunting increases causing increased PBF, which results in pulmonary over circulation. Over circulation of the lungs presents as tachypnea, sinus tachycardia, poor feeding, sweating with feeds, and failure to thrive, which may be mistaken for a respiratory illness. Typical examples of left-to-right shunt lesions include ventricular septal defect (VSD) and PDA.
Patients with left-to-right shunts usually present between 6 and 8 weeks of life. They present with tachypnea (not cyanosis) and have a history of poor feeding and/or weight gain. They may also have started out life feeding well and asymptomatic, but their feeding has worsened as their PVR dropped and pulmonary over circulation increased. On physical examination, the provider may or may not appreciate a murmur. A large, nonrestrictive VSD will not produce a murmur at all, since left ventricle (LV) and right ventricle (RV) pressures are equal. Small VSDs produce louder murmurs due to the pressure gradient from LV to RV. The murmur of a small VSD may become louder as PVR falls. Hepatomegaly is a prominent feature in large left-to-right shunts.
Right-to-left Shunts
Right-to-left shunts include defects that allow deoxygenated blood to pass from the right side of the heart into the systemic circulation resulting in cyanosis. Defects such as TOF are examples of this group. The physiology of TOF is defined by the large, nonrestrictive VSD and pulmonary stenosis even though TOF is described anatomically as pulmonary stenosis, a large nonrestrictive VSD, overriding aorta and right ventricular hypertrophy. The stenotic pulmonary outflow tract offers higher resistance to blood flow than SVR and blood preferentially shunts right-to-left through the VSD. The degree of pulmonary stenosis determines the volume of right-to-left shunting and thus the degree of cyanosis. The murmur is determined by the degree of pulmonary stenosis, not the VSD. These infants present with normal feeding, weight gain, and vital signs but they are cyanotic. Dehydration may worsen cyanosis.
Right-to-left and Left-to-right Shunts (Total Mixing Lesions)
Total mixing lesions such as atrioventricular septal defects (AVSDs) may present with mild cyanosis and pulmonary overcirculation. These patients have right-to-left and left-to-right shunting causing both cyanosis and pulmonary over circulation. Presentation will also be affected by associated defects such as pulmonary stenosis, unbalanced ventricles (one ventricle larger than the other), and so forth. If either of these associated defects is present with AVSD, they will present as a ductal dependent lesion, rather than as pulmonary over circulation.
Triage. Age, vital signs, weight, color, and respiratory status will indicate acuity of most congenital heart lesions (Table 94.3).
Initial Assessment/H&P. Newborn infants with significant congenital heart malformations may be completely asymptomatic until the DA closes or PVR drops. Large population studies have shown that screening newborns using pulse oximetry combined with physical examination prior to hospital discharge can detect up to 82.8% of all cardiac defects. The most likely defects to be missed in the newborn nursery are left ventricular (LV) obstructive lesions. This is important because the infant who is discharged from the nursery with undetected CHD, is at increased risk for mortality and morbidity.
In the ED, history should focus on age at presentation, feeding patterns, weight gain, breathing patterns, and color changes. When the patient presents in shock, cardiac and noncardiac diagnoses must be considered. Cardiac lesions that present with shock include those dependent on the DA for systemic blood flow (LV obstructive lesions) or severe ductal dependent right ventricular outflow tract obstruction (RVOTO). In addition, identifying a genetic syndrome may shed light on likely cardiac diagnoses (Table 94.4).
TABLE 94.2
CONGENITAL HEART DISEASE: INITIAL DIAGNOSIS
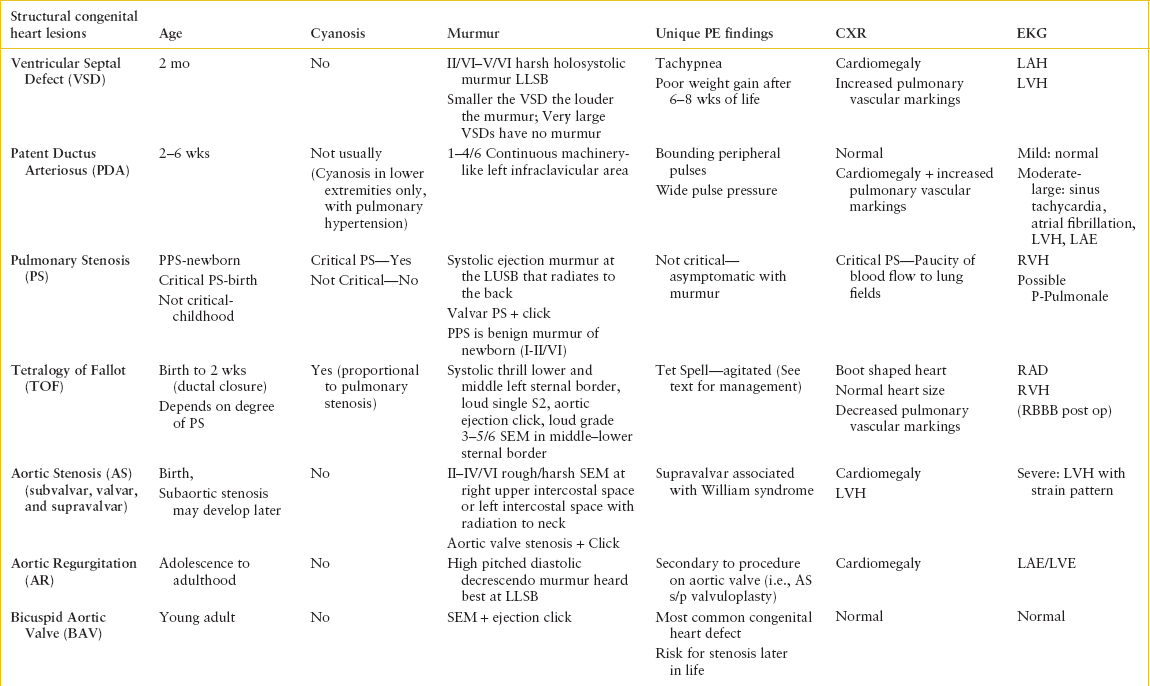

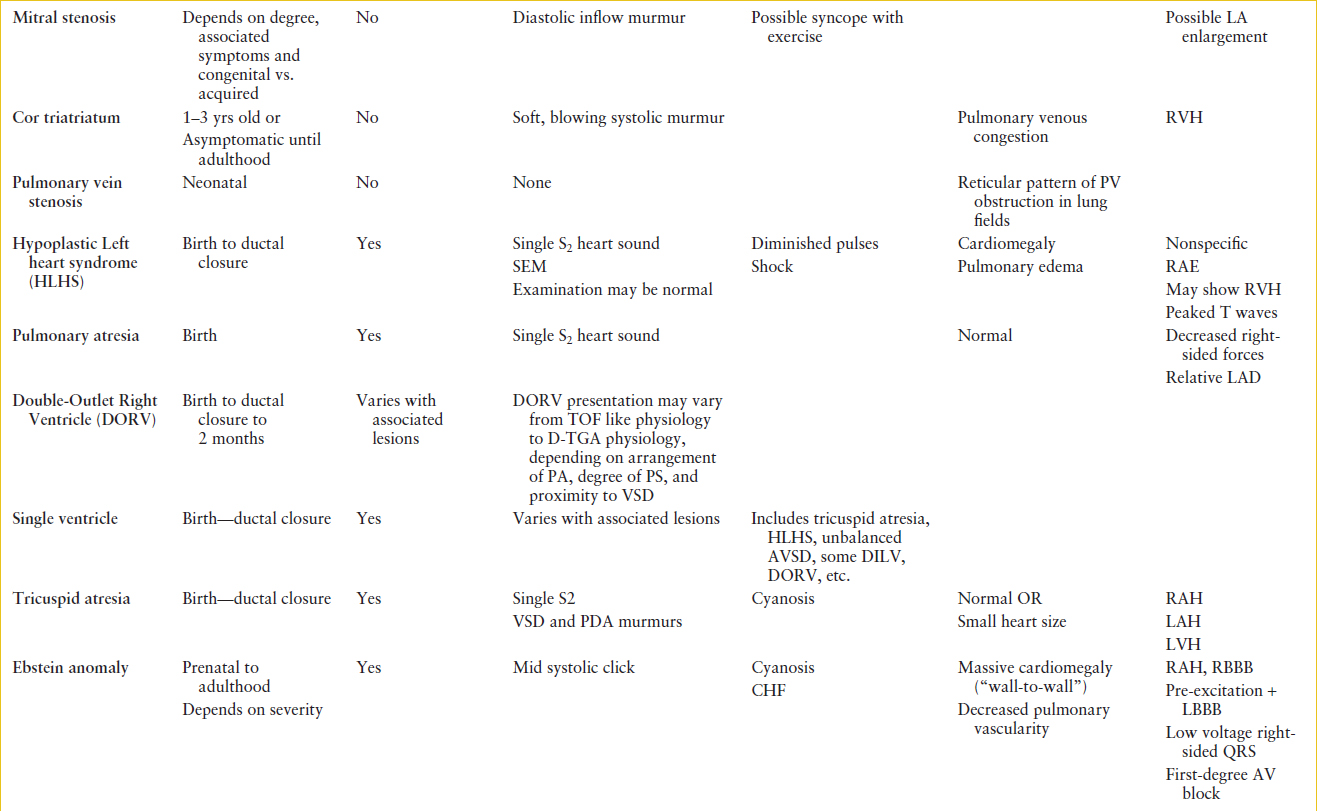
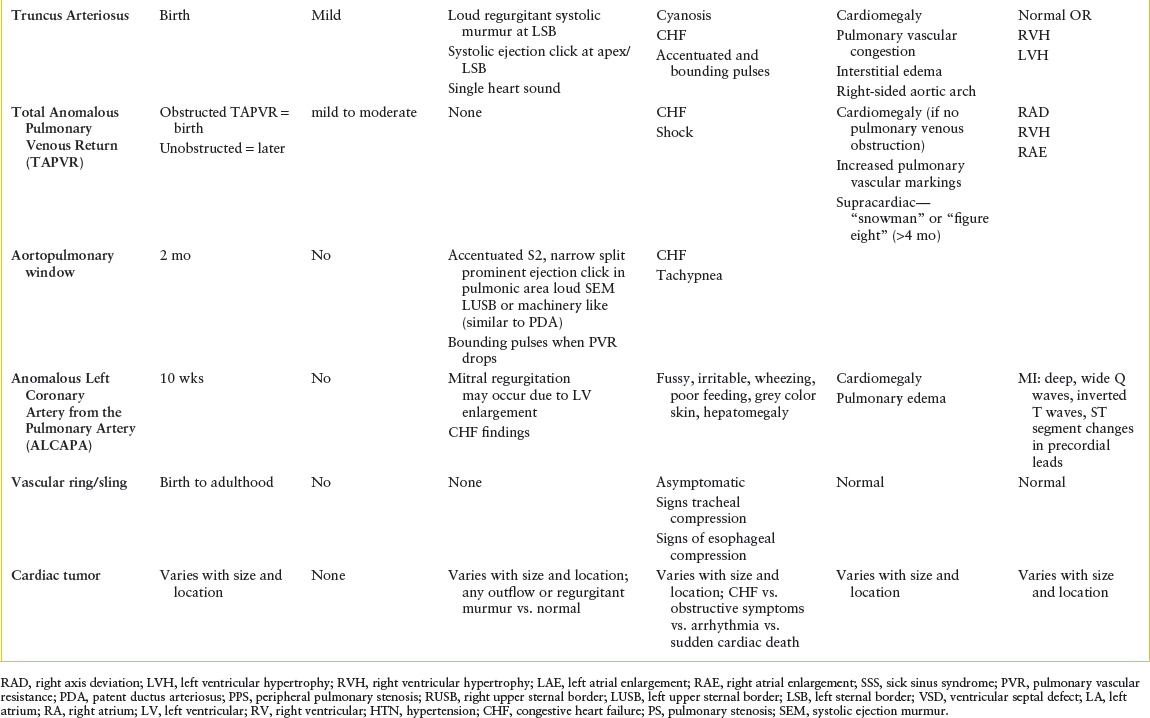
TABLE 94.3
AGE AND VITAL SIGNS OF INFANTS WITH CYANOSIS OR CHF/PULMONARY OVER CIRCULATION

Management/Diagnostic Testing of an Infant in Shock, Suspected Ductal Dependent Lesion. While immediate attention to airway, breathing, circulation, and high-quality CPR are first steps in the management of a patient in shock, initiation of prostaglandin (PGE1) to re-establish ductal patency is a lifesaving therapy. The dose of PGE1 is 0.05 to 0.1 mcg/kg/min via intravenous (IV) or intraosseus (IO) line. Side effects of PGE1 include hypotension, apnea, fever, and rash. Titrate PGE1 until femoral pulses are palpable or oxygen saturations improve. Effect should be seen within 30 minutes.
Endotracheal intubation and mechanical ventilation decrease the work of breathing by reducing cardiac demands and guard against apnea caused by PGE1. Epinephrine and other drugs for resuscitation should be drawn up and ready for administration during endotracheal intubation, since there is a high likelihood of cardiac arrest during this procedure. Once the airway is secured, aim to maintain oxygen saturations at approximately 75%. Over ventilation and hyperoxygenation may cause systemic blood pressure to drop significantly since oxygen is a potent pulmonary vasodilator. Pulmonary vasodilation drops the PVR, thereby increasing shunting of systemic blood flow into the lungs, and thus causing systemic hypotension. If the blood pressure falls, check for over ventilation or oxygen saturations above 75% to 85%. If possible, lower saturations to control PBF and restore systemic BP.
Chest x-ray (CXR) is useful to assess heart size and pulmonary circulation or pulmonary venous congestion. It also shows the cardiac silhouette, thoracic and abdominal situs, and aortic arch sidedness. Cardiac rhythm should be monitored. EM physicians trained in emergency ultrasound may assess cardiac function. Electrocardiogram (EKG) with rhythm strip is the gold standard for arrhythmia diagnosis, and may also offer clues in structural heart disease. Refer to Table 94.2 for typical presenting signs and symptoms, CXR, and EKG findings on initial evaluation of suspected CHD. Table 94.5 outlines common issues in patients after cardiac surgery.
Laboratory evaluation should include venous or arterial blood gas (ABG), electrolytes, blood urea nitrogen (BUN), creatinine (Cr), ionized calcium, magnesium, glucose, complete blood count (CBC), liver function tests (LFTs), and coagulation studies. Blood tests reflect end-organ damage of the liver and kidneys.
TABLE 94.4
GENETIC CONDITIONS AND TYPICALLY ASSOCIATED CHD
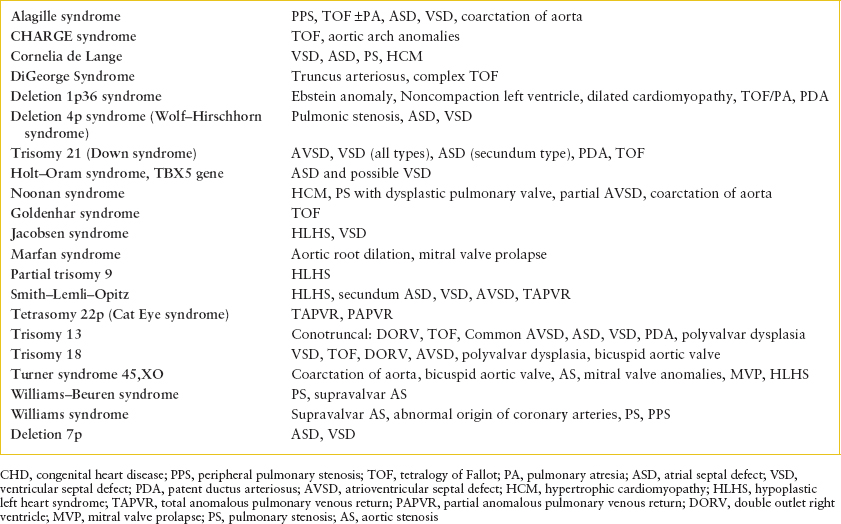
TABLE 94.5
CONGENITAL HEART DISEASE REPAIRS: PEARLS AND PITFALLS


Judicious fluid resuscitation based on clinical presentation may be employed. If the patient is anemic and cyanotic, red blood cell infusion may be used to expand intravascular volume. A urinary catheter should be inserted for fluid management. Initiate inotropic support as needed with dopamine 5 to 10 mcg/kg/min. Other inotropes to consider include epinephrine, norepinephrine, and milrinone.
As soon as cardiac pathology is suspected, consult cardiology. Consult cardiac surgery early if extracorporeal membrane oxygenation (ECMO) or surgical intervention is anticipated.
Management/Diagnostic Testing of an Infant with Cyanosis and Suspected Ductal Dependent Lesion. The presentation of cyanosis due to ductal dependent lesions is variable as ductal dependent lesions exist along a wide spectrum. If cyanosis is the predominant symptom, first administer 100% oxygen. Oxygen supplementation will not improve cyanosis due to cardiac causes but pulmonary causes should respond. Oxygen administration may be diagnostic as well as therapeutic in the stable patient. The hyperoxia test can help differentiate cardiac from pulmonary disease. After receiving 100% oxygen for 10 minutes, an ABG is tested. If the PO2 is greater than 150 mm Hg, it is normal. PO2 less than 100 mm Hg suggests cardiac etiology. Pulse oximetry should not be used for the hyperoxia test.
Treatment of cardiac cyanosis is the re-establishment of PBF by opening the DA using PGE1 (0.05 to 1 mcg/kg/min) as discussed above. Endotracheal intubation and mechanical ventilation will protect the infant from apnea, a common side effect of PGE1.
A hypercyanotic spell (Tet spell) is a cyanotic emergency, which may result in altered mental status, loss of consciousness, or death. Events are typified by hyperpnea, tachypnea, and agitation. It occurs when a patient with TOF or similar lesion involving RVOTO, experiences an event in which the muscular infundibular portion of the RVOT becomes diminishingly small, preventing blood flow to the lungs. All blood is then shunted right-to-left across the nonrestrictive VSD. The pulmonary stenosis murmur disappears. Extreme cyanosis ensues.
Treatment of a hypercyanotic event follows a stepwise progression. Initially, bring the knees to the chest, allowing the parent to comfort the patient in this position. Monitor closely. Knee to chest position in infants, or squatting in older children, increases SVR and decreases right-to-left shunting. Administer 100% oxygen. If the spell persists, morphine (0.1 mg/kg IM or IV) can be used to calm agitation. Normal saline bolus (5 to 10 mL/kg) ensures adequate preload and may be repeated if the patient is dehydrated. If these steps are not successful a continuous intravenous (IV) infusion of phenylephrine, an alpha agonist (0.5 to 5 mcg/kg/min), may be titrated to increase SVR. Propranolol, a beta-blocker, may be used to decrease heart rate and promote ventricular filling, but care should be exercised when administering this drug as hypotension may occur. If the spell still is not broken, general anesthesia and emergent surgery for placement of a systemic–pulmonary shunt or full repair is the next step. Ketamine 1 to 2 mg/kg IM or IV is an excellent drug for sedation for endotracheal intubation or other procedures.
After stabilization the patient should be admitted to an intensive care unit skilled in cardiac care. Chronic oral beta blocker therapy may be initiated in an attempt to decrease RVOT infundibular reactivity and thus prevent further spells. Definitive care is surgical repair or palliation with an aortopulmonary shunt.
Management/Diagnostic Testing of Infant Presenting with Left-to-right Shut and Pulmonary Overcirculation. Diuretic therapy is the mainstay of acute treatment for pulmonary overcirculation due to left-to-right shunt lesions. When used with afterload reduction the symptoms of overcirculation may be mitigated until the time of surgical repair. Hospital admission may be necessary for initiation of medical therapy, treatment of concurrent infections, or surgery if medical management is not effective. IV fluids and oxygen should be avoided in these patients, as either intervention will worsen pulmonary overcirculation.
Clinical Indications for Discharge or Admission. Any child newly diagnosed with hemodynamically significant CHD should be admitted to the hospital. Consultation with a pediatric cardiologist can guide this decision. Very ill patients such as those requiring a PGE1 infusion, should be admitted to an intensive care unit skilled in the care of sick neonates with heart disease. The safest mode of transport is with a skilled pediatric transport team.
Congenital Heart Disease: Postoperative and Other Considerations. For a reference on congenital heart defects, surgical repairs, common complications, and sequelae, see Table 94.5.
Some guiding principles will help EM providers navigate the specifics of a patient with CHD whose diagnosis is known. Red flags in CHD include age less than 2 months, especially with palliated or unrepaired defects, poor cardiac function, single ventricle anatomy, and arrhythmias. Special consideration should also be given to infants or older children with respiratory illness, especially respiratory syncytial virus, with palliative surgery or infants with pulmonary overcirculation.
Aortopulmonary shunts (modified Blalock–Taussig (BT), central shunts, etc.) may be larger or smaller than optimal, causing problematic over or under circulation of the lungs. The size of the shunts can be judged by oxygen saturation. Patients with excessive shunt flow will present with high saturations (>95%) and pulmonary edema. Patients with inadequate shunt flow due to shunt malfunction or decreased systemic blood pressure, or a small shunt present with cyanosis (saturations <75%).
A patient with BT shunt obstruction will present with cyanosis that may progress to cardiac arrest. History of CHD, worsening cyanosis, and lack of a shunt murmur raise the suspicion for shunt obstruction. Risk factors include shunt size <4 mm, very young age at the time of shunt placement, and low weight. The obstruction may be due to thrombus formation, often in the setting of dehydration or may be due to kinking of the shunt. No shunt murmur is audible on auscultation of the right or left infraclavicular area.
Treatment of shunt stenosis or clotting is administration of an IV normal saline bolus, heparin, and emergent surgical consultation. Shunt obstruction can be confirmed with an echocardiogram. Endotracheal intubation may be needed. Pressors may be used to augment systemic blood pressure to perfuse the shunt. Consider pulmonary hypertensive crisis in the differential diagnosis of a patient who presents with cyanosis and no shunt murmur. Pulmonary hypertensive crisis is treated with hyperventilation, oxygen, correction of acidosis, and sedation. Ketamine is a good choice for sedation if needed in these patients.
Infants who have undergone the first stage (Norwood Stage I) palliation for single ventricle are at high risk for sudden cardiac death (SCD). About 15% of these infants will die before the second palliative operation. Respiratory or other intercurrent noncardiac illnesses are very concerning and may be fatal. Nasogastric tubes should be placed with caution to avoid significant vagal stimulation. Coronary blood flow may be compromised in some patients, leading to acute heart failure and/or ventricular arrhythmias. Management of these patients in the ED should be expedited and cardiology consultation should be obtained prior to discharge. Caution should be used even when the infant is well appearing.
Postpericardiotomy syndrome (PPS) is a postsurgical syndrome of fever, pericardial and pleural inflammatory response, with effusions and malaise. It usually occurs one to several weeks after open-heart surgery, cardiac catheterization, or transvenous device implantation. The etiology is thought to be an autoimmune reaction. If pericardial fluid accumulates rapidly, it may present as cardiac tamponade.
If PPS is suspected, evaluate for leukocytosis and eosinophilia on CBC. Echocardiogram can detect pericardial fluid and assess for tamponade. PPS is treated with salicylates and pericardiocentesis if needed. Steroids are indicated if salicylates are not effective or the effusion is large.
Pleural effusions may be seen acutely after discharge from the hospital following any type of congenital heart surgery. Chylous or serous effusions may be chronic following Fontan procedure (caval pulmonary anastomosis) due to increased central venous pressures (lymphatic congestion).
Postoperative cardiac patients may present to the ED with wound infection, sepsis, endocarditis, and/or mediastinitis. Sepsis presents with fever or florid septic shock and occurs in 2.6% of patients after cardiac surgery. The usual pathogens are group A beta-hemolytic Streptococcus, Escherichia coli, Listeria monocytogenes, Staphylococcus aureus, and Pseudomonas aeruginosa. Endocarditis presents with nonspecific symptoms of fever, poor feeding, and malaise. High-risk situations for endocarditis include the presence of an abnormal aortic valve, residual VSD, TOF, and extensive repairs involving foreign material such as prosthetic valves. Atrial septal defects and right-sided defects are at lower risk for endocarditis. A new murmur may develop from dehiscence of a patch/conduit or turbulent flow caused by vegetations. Common pathogens implicated in endocarditis include Staphylococcus aureus, Streptococcus pyogenes, and Streptococcus viridans (Chapter 102 Infectious Disease Emergencies).
Mediastinitis is a serious postoperative complication that presents with local erythema, pain, induration, fluctuance, and purulent drainage from the sternotomy incision. This local infection may cause wound dehiscence and sternal instability. The most common pathogen causing mediastinitis is Staphylococcus aureus. Approximately 50% of patients with mediastinitis will have concurrent bacteremia.
When an infection is suspected, CBC, erythrocyte sedimentation rate (ESR), urinalysis, urine culture, and blood cultures should be obtained. Concern for sepsis, endocarditis, or mediastinitis warrants prompt treatment with empiric broad-spectrum IV antibiotics although cultures should be obtained from the wound or blood before antibiotics if the patient is stable. Judicious fluid resuscitation for septic shock depends on the heart disease. Give aliquots of 10 mL/kg crystalloid for fluid resuscitation in patients with poor ventricular function, volume overload, or significant AV valve regurgitation. Reassess frequently for signs of CHF. In all surgical complications, cardiothoracic surgery and cardiology should be consulted.
Cardiac Transplantation. Pediatric heart transplant patients presenting to the ED require special consideration. The ED physician must be well versed on common problems encountered in this group of patients. Concerns following transplant include rejection, infection, nonadherence, cardiac allograft vasculopathy (CAV), and issues arising from long-term immunosuppression.
Rejection, infection, and CAV may all present in a similar fashion. Pediatric transplant patients with these conditions present with subtle complaints including tachycardia, tachypnea, lethargy, irritability, abdominal pain, nausea, vomiting, and/or poor feeding. Abdominal pain is frequently endorsed in pediatric patients with rejection or symptomatic CAV. Atypical chest pain may also be a sign of CAV and of rejection (chest pain can occur as some patients partially reinnervate over time). Low-grade fever, malaise, and heart failure symptoms may also be present. Arrhythmias and conduction disturbances may be a sign of rejection or coronary artery vasculopathy. Physical signs may include jugular venous distension, hepatomegaly, new murmur, and a gallop.
Infections are a threat to the immunocompromised transplant patient. Prophylaxis against fungal (nystatin), CMV (ganciclovir, especially in CMV + donor), and protozoal (TMP–SMX) infections is used after transplant. In the first month following transplant, the greatest risk of infection is bacterial or fungal. In the second month, CMV and other viruses pose the greatest threat.
Long-term immunosuppression increases the transplant recipient’s risk for posttransplant lymphoproliferative disorder/malignancy, hypertension, and renal failure. In the adolescent age group, nonadherence is a leading cause of late rejection and death. Adherence to the medical regimen should be asked of all adolescent patients presenting to the ED. The transplanted patient should be carefully evaluated with these issues in mind when presenting to the ED for any reason.
Following a careful physical examination, B-type natriuretic peptide (BNP), chemistry, CBC, blood cultures, viral studies, CXR, EKG, and echocardiogram may aid diagnosis and treatment. Prompt consultation with the cardiac transplant service is recommended when these patients present to the ED.
For more information on complications specific to particular lesions or complications specific to particular procedures/surgical repairs refer to Table 94.6.
Adults with Congenital Heart Disease
As a result of advances in diagnosis and surgical management, most patients born today with CHD survive into adulthood. There are an estimated 1 million adults living with CHD in the United States of America. These patients require specialized care provided by cardiologists trained in this field. Commonly encountered complications in adults with congenital heart disease (ACHD) include poor cardiac function and atrial arrhythmias. Similar to children with CHD, history should elicit information about diagnosis, baseline cardiac function, concurrent medical history, medication, and previous problems encountered.
Intraatrial reentrant tachycardia (IART) is a variation of atrial flutter where the reentrant circuit is caused by scarring in the atrium from previous surgeries. Often the rate of IART is relatively slow and the amplitude of the P waves is low, making this arrhythmia hard to detect. The heart rate in IART may be 80 to 86 BPM and may look like sinus rhythm. Patients with single ventricle palliation typically do not tolerate any nonsinus rhythm, even if its rate is controlled. Adenosine blocks AV conduction transiently but usually does not terminate tachycardia. Due to the low amplitude of the P waves, continuation of IART during adenosine administration is often missed. Synchronized cardioversion is typically the treatment of choice.
Sudden death can be a complication after repair of CHD. Risk increases with increasing age of the patient, complexity of the repair, and with poor ventricular function. Long term, the highest risk lesions include TOF, aortic stenosis, transposition of the great arteries, coarctation of the aorta, AVSD, pulmonary stenosis, and anomalies that undergo the Fontan procedure. Repaired VSD and PDA ligation are low risk procedures with respect to SCD.
ARRHYTHMIC EMERGENCIES
Goal of Treatment
The primary goal of treatment in a cardiac arrhythmic emergency is rapid recognition and correction of unstable arrhythmias while simultaneously documenting the rhythm on EKG. A secondary goal is to identify patients with subtle signs of aborted SCD.
TABLE 94.6
LESION SPECIFIC COMPLICATIONS

CLINICAL PEARLS AND PITFALLS
• Any incessant tachycardia can cause CHF and diminished LV function.
• In dilated cardiomyopathy (DCM), it may be difficult to discern which came first: tachycardia or DCM.
• Arrhythmias are not well tolerated in the setting of structural heart disease, especially single ventricle or in those who have undergone palliative repairs.
• Arrhythmias are not well tolerated in patients with poor LV function.
• Adenosine may be diagnostic as well as therapeutic.
• Never give adenosine in a wide complex irregular rhythm.
• In heart transplant patients, use adenosine with extreme caution at one-third to half the normal recommended dosage. Be prepared to pace in the event of asystole.
• Ventricular tachycardia (VT) may look narrow in infants.
• Long QT syndrome (LQTS) presents more frequently with sudden death as the first symptom in pediatrics than in adults.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







