3. Left heart obstruction (mitral valve area <2 cm2, aortic valve area <1.5 cm2, or peak left ventricular outflow tract gradient >30 mm Hg by echocardiography)
4. Reduced left ventricular function (ejection fraction <40%)
Risk estimation was done by assigning one point for each predictor. The estimated risks for a cardiac event with 0, 1, and >1 points are 5%, 27%, and 75%, respectively, and this risk prediction model is the most widely used to date.6 Cardiac events were defined as pulmonary edema, sustained tachyarrhythmia, or bradyarrhythmia requiring treatment, stroke, cardiac arrest, or cardiac death.
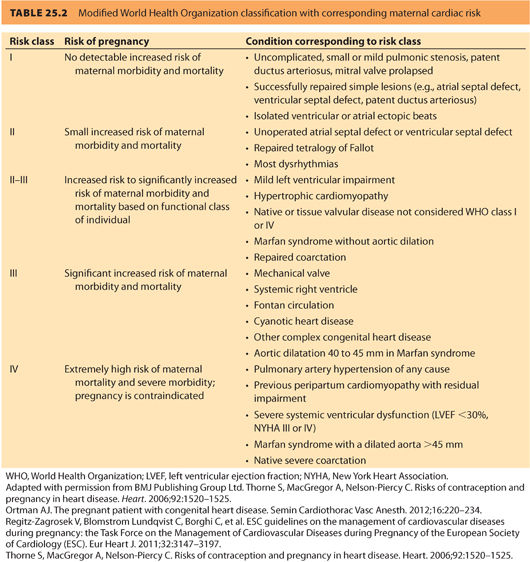
In 2011, the European Society of Cardiology (ESC) generated a report from the Task Force on the Management of Cardiovascular Diseases during Pregnancy, which considers both acquired cardiac disease and CHD. Table 25.2 is a summary of risk classification from this report using the World Health Organization (WHO) classification system.6 A 2014 prospective validation study indicated that the WHO classification system more accurately predicted events than either the CAREPREG or ZAHARA risk models.9
C. Neonatal risk
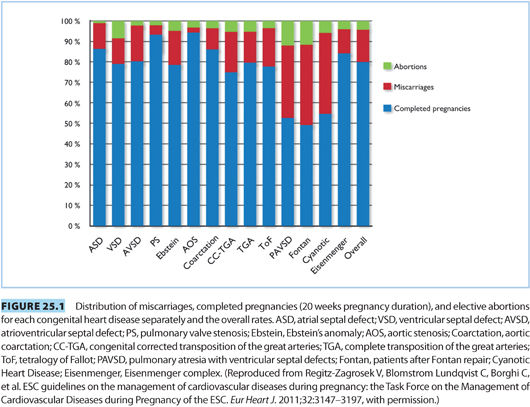
Considerations must be made for both the risk of neonatal disease and for failure of the pregnancy. Figure 25.1 indicates the distribution of completed pregnancies, elective abortions, and miscarriages by specific maternal congenital cardiac defect. Incidence of preterm delivery of neonates born to mothers with CHD is elevated, with an incidence of 16% to 19%. Rates of neonatal intensive care unit admission, intraventricular hemorrhage, small for gestational age, and neonatal mortality are also all elevated.10,11 Neonates born from mothers with CHD have a tenfold elevated risk of suffering CHD with an incidence of 3% to 10%.12 Genetic testing may be useful in select patients with increased risk profile.
D. Obstetric delivery considerations
1. Vaginal delivery is typically preferred over cesarean delivery (CD) because of lesser blood loss and fluid shifts, a lower incidence of postoperative infections and pulmonary complications, as well as reduced metabolic demands and stress response.8
2. CD has been suggested for the following cardiac conditions: Marfan syndrome with aortic root involvement, severe aortic stenosis, aortic dissection, recent myocardial infarction, and severe heart failure during a previous delivery.13,14 The principal rationale for these recommendations is avoidance of the hemodynamic stress of labor and delivery, although one could speculate that the afterload and hemodynamic stress reduction associated with epidural analgesia might be favorable for situations such as Marfan syndrome with aortic insufficiency and aortic dissection.
II.Valvular heart disease: congenital and acquired
Valvular heart disease accounts for approximately 15% of cardiac disease among parturients, with rheumatic heart disease being most prevalent.15 Stenotic abnormalities carry the highest risk for both the mother and developing fetus.1,15 Regurgitant lesions are better tolerated, but can worsen to the point of increasing the NYHA class. The severity of the valvular lesion and underlying cardiac function correlate with the risk of cardiac complications during pregnancy and delivery.3 Echocardiography constitutes the gold standard for both diagnosis and grading the severity of valvular lesions.16
A. Mitral stenosis
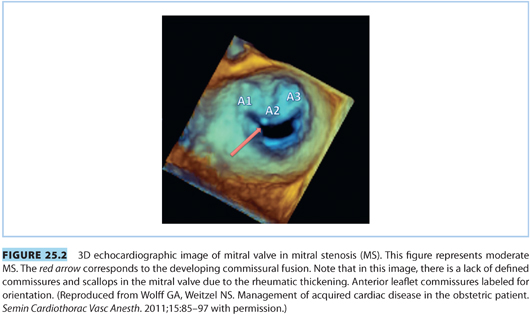
Mitral stenosis (MS) remains fairly common worldwide and most often follows childhood rheumatic fever. However, the incidence of MS has declined significantly in developed nations as a result of recognition and treatment of streptococcal disease.13 The natural history of MS is a slow progression of disease and symptoms over 20 to 30 years. The mitral valve leaflets thicken, calcify, and fuse near the chordae or commissures (see Fig. 25.2) to induce a stenotic flow pattern.
1. Pathophysiology
a. Typical symptoms or signs of severe MS include chest pain, dyspnea, palpitations, pulmonary edema, hemoptysis, and thromboembolism.
b. The stenotic valve prevents normal left ventricle filling and leads to enlargement of the left atria, elevated pulmonary venous and arterial pressures, and eventually to right-sided heart failure often with associated tricuspid valvular insufficiency.
c. Left ventricular stroke volume decreases along with the cardiac output.
d. Severity is judged by mitral valve area obtained by echocardiography.16 Normal mitral valve area is 4 to 6 cm2 with mild stenosis typically defined as a valve area of 1.5 to 3 cm2, moderate stenosis as 1.1 to 1.5 cm2, and severe stenosis as <1.0 cm2. Severe lesions typically require surgery, which can usually be done at a relatively low risk to both mother and fetus during the first trimester of pregnancy.
2. Peripartum considerations. The “hyperdynamic” cardiac state of pregnancy is poorly tolerated by women with severe MS.16
a. Increased plasma volume can cause pulmonary edema and worsen left atrial enlargement.
b. Tachycardia decreases left ventricular diastolic filling time through the stenotic valve.
c. Atrial fibrillation (AF) is common in MS, and this can be highly detrimental because the atrial “kick” may account for as much as 30% of the left ventricular stroke volume in patients with MS.16 Medical management of AF typically includes ventricular rate control by using β-adrenergic blocking agents, unless there is a contraindication; yet, a resting HR of approximately 90 to 100 bpm is considered normal during pregnancy.
d. Patients with severe disease have improved outcomes with intervention prior to pregnancy. Typical interventions include surgical mitral valve commissurotomy, valve replacement, or percutaneous mitral commissurotomy.16–19
3. Anesthetic management
Management should be based on the severity of the lesion, which depends on the mitral valve area and the hemodynamic stability of the patient.19 Most obstetric anesthesiologists advocate early pain control using either IV opioids or epidural analgesia for labor and vaginal delivery.20–22 Second stage of labor increases cardiac preload and cardiac output as a result of autotransfusion from the uterus and relief of aortocaval compression. These alterations in hemodynamics and cardiac preload can cause acute pulmonary edema.
Combined spinal-epidural (CSE) technique, where intrathecal opiates are given early in the first stage of labor followed by gradual titration of dilute epidural local anesthetic as labor progresses to the second stage (to cover T10–S4), provides an excellent hemodynamic profile for most patients.19
a. Epidural anesthesia is ideal for prolonged second stage vaginal deliveries where instrumentation is required. Often the epidural infusion during labor will provide adequate anesthesia for forceps placement. If the epidural block is inadequate, administration of epidural fentanyl along with small doses of 2% lidocaine or 3% 2-chloroprocaine can provide surgical blockade in a timely and safe manner.19
b. Reductions in SVR from sympathetic blockade should be treated preferentially with cautiously titrated phenylephrine and judicious volume expansion, unless the patient is relatively bradycardic (HR <70 bpm). In this situation, ephedrine may be preferable.
4. For CD, epidural anesthesia is the preferred approach if time allows.1,19
a. “Single-shot” spinal techniques should be avoided in both moderate and severe MS because abrupt changes in SVR are poorly tolerated.
b. Slow titration of 2% lidocaine combined with fentanyl through the epidural catheter will produce surgical blockade (T4–T6 level). Titration of small doses of phenylephrine (50 to 100 μg) as needed will counteract the expected hypotension from sympathectomy. Systolic BP should ideally exceed 100 mm Hg, although the potential exists to exacerbate pulmonary hypertension. If echocardiography in the second or third trimester suggests pulmonary hypertension or signs of right heart “overload” (i.e., dilation of right ventricle and/or atrium, often with tricuspid regurgitation), then placement of a pulmonary artery catheter (PAC) for labor and delivery should be considered.
c. The blood volume reduction that accompanies vaginal delivery and CD are beneficial to the patient with MS, as long as adequate cardiac preload is maintained.
5. General anesthesia. Consideration of the above hemodynamic goals should be used in choosing induction and maintenance anesthetics.1,19
a. Stress response suppression during induction and through the predelivery period, using opiates such as remifentanil (0.5 to 1 μg/kg) and a short-acting β-adrenergic blocker such as esmolol (30 to 50 μg/kg) at induction, should be considered. Etomidate 0.2 to 0.3 mg/kg is a good choice for induction, typically accompanied by succinylcholine 1 to 1.5 mg/kg in a rapid-sequence technique accompanied by cricoid pressure.
b. Drugs that may produce tachycardia should probably be avoided (e.g., glycopyrrolate, atropine, meperidine, pancuronium, ketamine).
c. Full stomach and aspiration prophylaxis are the same as for CD under general anesthesia without mitral stenosis.
d. Methergine, prostaglandins, and oxytocin should be used cautiously because these drugs may alter systemic or pulmonary pressures leading to hemodynamic compromise.22
6. Invasive monitoring including arterial and central venous or pulmonary arterial pressure monitoring should be considered for high-risk patients. The risks of placing a PAC, especially dysrhythmias, are significant, but the information gained in patients with severe MS may be beneficial to management during labor.19,20,23 If general anesthesia is selected, consideration should be given to the use of transesophageal echocardiography to facilitate decision making about fluid replacement and/or inotropes and vasopressors.
CLINICAL PEARL Anesthetic and hemodynamic goals in MS:
• Maintain hemodynamics close to the patient’s normal state. This goal is facilitated by adequate pain control and by avoiding hypoxia, hypercarbia, and acidosis.
• Preserve sinus rhythm (if present) and treat AF aggressively.
• Avoid aortocaval compression. Monitor and maintain adequate cardiac preload without causing pulmonary edema.
• Provide supplemental oxygen.
• Avoid reductions in SVR.
B. Aortic stenosis
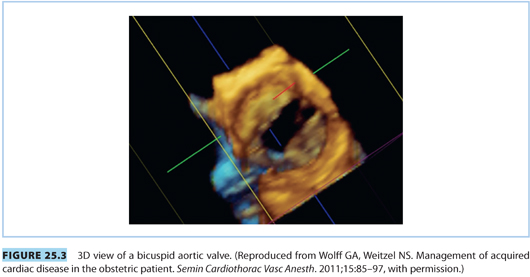
Severe aortic stenosis (AS) is rare among parturients. Rheumatic heart disease is the most common acquired cause of AS, whereas a bicuspid aortic valve is the most common congenital cause (see Fig. 25.3). Left ventricular hypertrophy compensates for AS by offsetting the increase in left ventricular wall tension imposed by increased left ventricular pressures. Unlike MS, symptoms of AS appear late in the course of disease and lead to high mortality.14,24 Patients with severe AS should have surgical correction prior to pregnancy.1,21
1. Pathophysiology
a. Symptoms of severe AS include syncope, dyspnea, angina pectoris, and fatigue.1
b. Patients are usually asymptomatic until the valve area is reduced to 1.0 cm2, which is roughly one third the normal valve area of 3 to 4 cm2.16,25
c. Severe AS is often defined as a valve area less than 0.7 cm2, which typically is associated with a transvalvular mean pressure gradient greater than 50 mm Hg derived from echocardiographic measurements.16,25
2. Peripartum considerations
a. Pregnancy is usually tolerated by women with mild to moderate AS.8,14
b. Patients with severe AS are unable to accommodate increased blood volume and tachycardia, and often experience worsening of symptoms including angina pectoris and dyspnea. These women have little cardiac reserve during pregnancy and have a high mortality rate of 15% to 17%.1,14,24
3. Anesthetic management1,22,24
Vaginal delivery is preferred for mild and moderate AS, whereas CD is often chosen for patients with severe AS. Keeping the HR below 90 bpm improves ventricular emptying against a fixed obstruction, although lower HRs may be required to avoid subendocardial ischemia in patients with severe AS and marked LVH. Tachycardia can induce left ventricular subendocardial ischemia, reduce left ventricular ejection fraction, and increase myocardial oxygen consumption. These women also have a limited capacity to increase left ventricular stroke volume, so the usual LV preload augmentation that accompanies slower HRs is impaired. Consequently, an HR below 70 bpm may place these patients at risk for critically low cardiac output. Left ventricular hypertrophy places the myocardium at risk for ischemia under situations of high myocardial oxygen demand (typically manifested in AS as excessively high left ventricular systolic pressures or tachycardia) or when SVR is reduced and coronary perfusion pressure (i.e., diastolic blood pressure minus left ventricular diastolic pressure) is compromised.
a. Single-shot spinal anesthesia is contraindicated for women with severe AS and relatively contraindicated for those with moderate AS.22
b. Epidural or continuous spinal approaches for labor have been successfully carried out with slow titration of both an opioid and a local anesthetic agent.26 Epinephrine should be avoided because of the risk of causing tachycardia with intravascular injection.
c. As in MS, early analgesia is beneficial. CSE with an intrathecal opioid followed by epidural titration of a dilute local anesthetic solution as labor progresses is ideal. Management of prolonged second stage instrumented deliveries is the same as for MS.
4. For general anesthesia, slow titration of opioids and use of etomidate constitute an excellent approach. As with MS, avoiding tachycardia is important, so glycopyrrolate, atropine, pancuronium, and ketamine should be avoided. Propofol should be used cautiously, if at all, due to reductions in SVR, cardiac preload, and myocardial depression.14
5. CD considerations are similar to MS except that AS patients are more afterload dependent and may tolerate the sympathectomy induced by spinal or epidural anesthesia even more poorly than MS patients. Both MS and AS patients also tolerate preload reduction poorly, but AS patients are probably more likely to experience it, and this may become clinically significant even with slow onset of an epidural anesthetic.
6. Invasive monitors
a. Consider arterial catheter placement to closely monitor changes in blood pressure.
b. Central venous pressure or pulmonary artery catheter. Use of PAC monitoring is controversial, and placement decisions should be made on a case-by-case basis.1,7,14,15,22 Tight volume control is recommended in these patients, but introduction of the PAC creates some risk of dysrhythmias, which can be life threatening. Hypovolemia should probably induce greater concern with moderate or severe AS than pulmonary edema because preload reduction can severely reduce cardiac output. Central venous pressure (CVP) monitoring may be an adequate substitute for the PAC to help monitor the volume status in the setting of moderate to severe disease.1,8 Attempts should be made to keep CVP in the high normal to slightly elevated range (8 to 12 mm Hg). With severe AS, neither CVP nor pulmonary artery occlusion pressures reliably reflect left ventricular preload, and the use of transesophageal echocardiography (TEE) should be considered for this purpose if general anesthesia is utilized.
CLINICAL PEARLAnesthetic and hemodynamic goals in AS during labor and delivery:
• Maintain hemodynamics close to the patient’s normal state. Adequate pain control helps avoid alterations in hemodynamics.
• Avoid tachycardia. Maintenance of sinus rhythm is critical.
• Avoid reductions in SVR and maintain preload to the heart. Aortocaval compression is not well tolerated.
• Avoid myocardial depressant agents during general anesthesia.
C. Mitral regurgitation
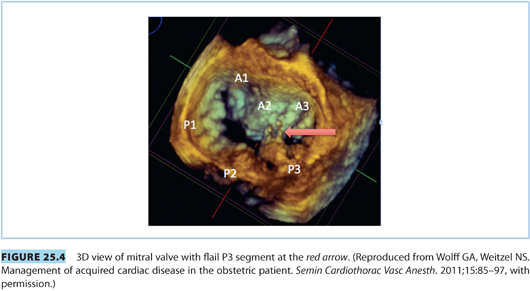
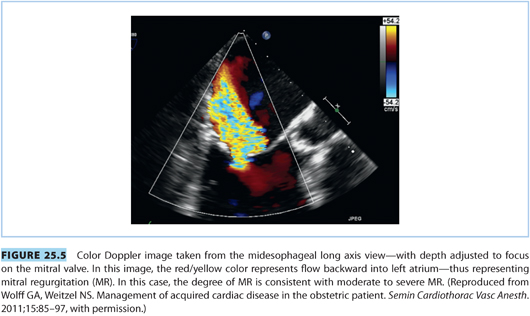
Consideration of mitral regurgitation (MR) requires division into acute MR and chronic MR. The most common causes of acute MR during pregnancy are bacterial endocarditis, trauma, papillary muscle rupture (see Fig. 25.4), and prosthetic valve dysfunction. Chronic MR is more common and usually results from myxomatous degeneration or rheumatic disease. Echocardiographic imaging is the standard diagnostic modality, and a case of moderate to severe MR is shown in Figure 25.5. MS and MR may coexist in pregnant patients. Women with severe symptomatic MR should consider mitral valve repair prior to conception.21
1. Pathophysiology
a. Acute MR results in an acute volume load into the left atrium across the incompetent mitral valve. This results in elevated pulmonary pressures and reduction in forward flow. If the patient survives the acute insult, pulmonary edema and right heart failure may occur and emergency valve surgery is often required.22
b. With chronic MR, the lesion develops over time and the left atrium and pulmonary venous system are able to accommodate the increased blood volume by enlarging. This predisposes the patient to atrial fibrillation. Elevated pulmonary pressures are much less common in chronic MR compared with acute MR, but certainly can occur with severe chronic MR. Pulmonary congestion is common.22
c. AF is better tolerated in MR as compared to either MS or AS because left ventricular preload is better maintained.8,15
2. Peripartum considerations. MR is well tolerated in pregnancy, unless the patient has severe MR or is symptomatic before pregnancy.3
a. Afterload reduction is the treatment for MR, so the decreased SVR that accompanies pregnancy is helpful. Moderate tachycardia is also helpful because it limits the time for regurgitation, so the increase in HR seen with pregnancy is similarly helpful.
b. Hypertension during pregnancy should be aggressively treated because increased afterload will increase the regurgitant fraction.24
c. The risk of thrombus formation is increased as a result of the enlarged left atrium and the hypercoagulability of pregnancy. Thrombus is found in up to 20% of patients.1
D. Mitral valve prolapse
Mitral valve prolapse (MVP) is a subset of mitral valve disease that most often involves early myxomatous degeneration with minimal MR in women of childbearing age, but advanced disease with severe MR is possible. This lesion is quite common and is found in 12% to 17% of women.24 MVP may predispose the patient to dysrhythmias and may be associated with other systemic diseases such as Marfan syndrome. In the absence of concomitant disease, there are no specific alterations in the anesthetic management of these patients because there are rarely any cardiac complications that arise from prolapse alone.24 In contrast to patients with most forms of advanced MR, patients with early MVP typically have less MR in the presence of high preload, high afterload, and low HR.
E. Aortic insufficiency
Aortic insufficiency (AI) occurs more commonly than AS, and rheumatic disease is the causative agent in the majority of patients. Patients with rheumatic AI often have concomitant mitral valve disease. AI can also be present in patients with congenital bicuspid valve disease or with collagen vascular disorders.24 Acute AI can result from endocarditis, or rarely from trauma. These patients are critically ill and typically require urgent surgical correction. There have been reported cases of medical management of acute AI until CD could be performed, but it is rare for those patients to survive.27
1. Pathophysiology
a. AI increases left ventricular diastolic volume. Over time, this causes significant ventricular dilation and eccentric hypertrophy. This typically precedes the onset of symptoms, which include dyspnea, diminished exercise ability, pounding in the chest, and orthopnea.1
b. Progression of disease causes left ventricular failure, reduced ejection fraction, and further increases in end-diastolic ventricular volume.
c. In the absence of pregnancy, symptoms typically occur 10 to 20 years after the onset of rheumatic disease.24
2. Peripartum considerations. Mild to moderate AI is well tolerated during pregnancy.
a. The tachycardia associated with pregnancy reduces the regurgitant fraction and left ventricular diastolic volume. The reduction of SVR associated with pregnancy also promotes forward flow out of the left ventricle.
b. Patients with severe AI who have symptoms of heart failure prior to pregnancy have diminished cardiac reserve. Onset of pregnancy-related hypertension or signs of congenital heart failure (CHF) should prompt treatment with afterload reduction, diuretics, and/or inotropic agents.21,24
CLINICAL PEARLAnesthetic and hemodynamic goals in MR and AI:
• Afterload reduction is beneficial. Avoid increases in SVR, so early and effective analgesia during labor is recommended.
• Avoid and treat atrial fibrillation aggressively.
• Slight tachycardia is preferred (target HR of 80 to 100). Bradycardia is poorly tolerated.
• Maintain preload and avoid aortocaval compression.
• Myocardial depression should be avoided.
3. Anesthetic management (management of both MR and AI). Patients with regurgitant lesions can be managed safely using either general or neuraxial anesthesia.
a. Epidural analgesia or CSE for labor is preferred by many because early administration during the first stage of labor permits titration with a combination of an opiate and local anesthetic agent, which facilitates cautious and titrated hemodynamic management.1
b. Early analgesia helps prevent pain-induced elevation in catecholamine levels that will increase SVR and therefore increase the regurgitant fraction.
c. Invasive monitoring should be reserved for those patients with severe and symptomatic MR or AI because risk of placement may outweigh the benefit.1 TEE should be considered with severe MR or AI if general anesthesia is used.
4. Cesarean delivery. Epidural anesthesia for CD is preferred, but spinal or general anesthesia may be acceptable alternatives.1,24
a. Spinal anesthesia can be used, but the abrupt changes in hemodynamics may not be tolerated by patients with more severe disease.
b. Bradycardia is poorly tolerated and can push the patient into cardiovascular compromise. Treatment of hypotension should be carried out preferentially with ephedrine to avoid bradycardia, and promote slight tachycardia.
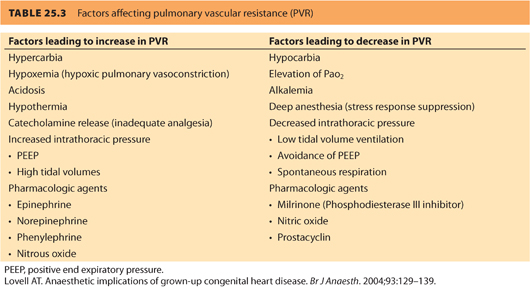
c. Avoiding increased PVR in severe MR is important (see Table 25.3). Acute increases in PVR can reduce forward flow and induce right ventricular failure.
5. General anesthesia. Overall hemodynamic goals are similar, and the agents used should ideally promote slight tachycardia and reduce SVR without producing myocardial depression.1,24
a. Induction with ketamine and use of glycopyrrolate to prevent or treat bradycardia.
b. Agents such as propofol can be used, but slow titration is recommended to avoid significant myocardial depression or preload reduction.
c. AF should be avoided and aggressively treated.
III.Congenital heart disease in the adult parturient
Adult patients with CHD now represent the majority (60% to 80%) of obstetric patients with cardiac disease.28 Significant improvements in surgical techniques have allowed many patients (85% of children with CHD) to not only survive to adulthood, but also have relatively normal function. In 2000, the 32nd Bethesda Conference report generated from the American College of Cardiology indicated that approximately 85% of patients with surgical repair and CHD survive to adulthood.29 It was estimated that 800,000 patients with adult congenital heart disease (ACHD) were living in the United States in 2000, with an additional 8,500 corrected each year.30,31 This report highlighted the importance of developing a model for seamless transitions of care for patients who are cared for at pediatric heart centers who now must transition into the adult population and adult hospitals.
This population can pose obstetric and anesthetic challenges as the physiology of pregnancy interacts with each patient’s corrected or uncorrected cardiac pathology. Figure 25.1 demonstrates the distribution of completed pregnancies, elective abortions, and miscarriages by congenital cardiac lesion.6 A full discussion about pre-pregnancy risk assessment and counseling exceeds the scope of this chapter, and typically, the anesthesiologist will not be involved with these aspects of care. However, when the patient with CHD presents in labor, it is important to have an understanding of the relative risks for the different lesions (see Table 25.2). CHD lesions will be discussed in two broad categories: cyanotic lesions and lesions resulting in left-to-right shunts.
A. General peripartum considerations. For patients with either cyanotic lesions (often with right-to-left shunts) or lesions with left-to-right shunts, who received definitive surgical repair as a child and have good functional results from the surgery, pregnancy is often well tolerated and can be managed like a normal parturient. Those patients presenting with partial correction, palliative procedures, uncorrected lesions, and residual cardiac defects need to be managed according to their current cardiovascular physiology. Some key considerations for determining the level of concern when approaching CHD patients are as follows32–40:
1. Evidence of congestive heart failure (even if compensated). If present, 30% of patients will have functional decline during pregnancy compared to 5% in those without CHF.
2. Presence of cyanosis. If cyanotic (oxygen saturation less than 90%), more than 50% of patients will deteriorate during pregnancy, whereas only 15% of acyanotic patients will show signs of cardiovascular deterioration.
3. Pulmonary hypertension. These patients are in the highest risk category for death or severe cardiac complications with pregnancy.
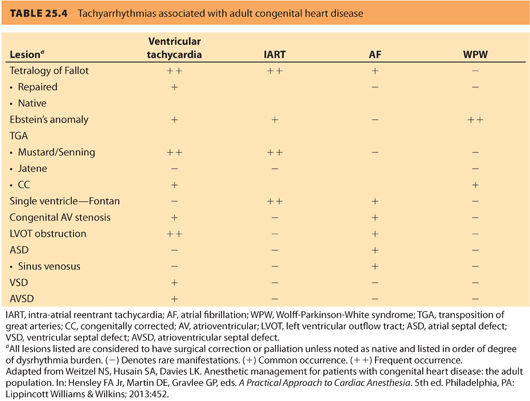
4. Presence of dysrhythmias. Ventricular and atrial dysrhythmias are extremely common in ACHD patients and account for nearly 50% of emergency hospitalizations.41 Dysrhythmias in this population may be treated with anti-arrhythmics, implantable devices, ablative procedures, or combinations of the earlier mentioned. Table 25.4 outlines incidence of dysrhythmia based on a specific congenital lesion.
5. Corrected single ventricle or systemic right ventricle. These patients should be considered at intermediate to high risk, and level of concern should be based on functional ability and type of surgical correction prior to pregnancy.
6. Presence of residual ventricular septal defect. Changes in SVR during pregnancy can alter the shunt physiology in an asymptomatic patient, typically by decreasing SVR to lessen a left-to-right shunt, or to potentially convert a left-to-right shunt or incipient Eisenmenger complex to a right-to-left shunt with cyanosis.
Severity and functional ability impact the type of anesthetic chosen. These patients will usually be followed by a cardiologist, and consultation should be obtained to gain information on the functional ability and cardiac status. After determining functional status and possible residual cardiac pathology, the anesthesiologist can tailor the individual anesthetic to achieve the appropriate hemodynamic goals.32 Current echocardiographic data should be sought to gain information on current cardiac function and anatomy.
B. Cyanotic lesions
1. General considerations. Some broad principles apply to anesthetic management of cyanotic congenital heart lesions, particularly those with residual defects.2,32–34,38–40
a. Cyanotic lesions have some element of right-to-left shunt even after surgical repair. The degree of this shunt determines the level of cyanosis present. Caution should be taken with sedative medicines because decreasing ventilation can increase PVR and exacerbate cyanosis by increasing right-to-left shunt.
(1) Right-to-left shunting reduces the uptake of inhalational anesthetics and prolongs inhalation induction. Conversely, the onset of IV induction hastens.
(2) Nitrous oxide may elevate pulmonary artery pressure and should be used cautiously.
b. Risk of air embolus. Extreme care should be taken to avoid an air embolus. Saline should be used for loss of resistance during epidural placement, not air, because air entrainment into an epidural vein can easily cross into the systemic circulation. IV lines should be aggressively de-aired and monitored.
c. Systemic vascular resistance. Changes in SVR disrupt the balance between pulmonary and systemic circulations to change the shunt. All anesthetic medications should be slowly titrated to prevent rapid changes. This holds true for both neuraxial and general anesthetics.
(1) Single-shot spinal anesthetic techniques are generally contraindicated because the quick onset of sympathectomy is poorly tolerated.
(2) Administration of antibiotics (e.g., vancomycin) may reduce SVR, particularly if administered quickly.
(3) Choice of anesthetic induction drug is not as important as the manner and vigilance used by the anesthesiologist in managing hemodynamics.
(4) Phenylephrine is generally the vasopressor of choice unless it is highly likely that the primary cause for hypotension is myocardial depression, in which case ephedrine or epinephrine should be considered.
(5) Interventions that could decrease PVR, such as increases in mixed venous O2 (typically via high Fio2) and modest degrees of respiratory alkalosis, are encouraged to reduce shunting.
d. Invasive monitoring
(1) Previous surgical procedures, such as Blalock-Taussig shunts using the subclavian artery, compromise blood flow to the ipsilateral upper extremity and will affect the location for arterial line placement.
(2) Central venous catheters should be reserved for the most symptomatic patients because an increased risk of thrombus and stroke PACs are anatomically difficult or impossible to place and are seldom helpful in patients with cyanotic cardiac lesions.
(3) TEE may be the most useful real-time monitor of cardiovascular status, especially when administering general anesthesia.
e. Aortopulmonary collaterals. Unrepaired complex cyanotic patients often develop aortopulmonary collaterals via the bronchial arteries. This causes a left-to-right shunt through the pulmonary circulation, which can potentially compete with the coronary arteries and compromise myocardial perfusion.40 Situations causing increased SVR, such as pain during labor, may increase this left-to-right shunt.
f. Prolonged second stage labor. Earlier transition to instrumented delivery may help to reduce the hemodynamic stress on an already abnormal cardiovascular system.42 Slowly and carefully titrated epidural analgesia for labor supplemented by pudendal nerve blockade for delivery (to avoid excessive epidural spread and decreased SVR) may optimize pain control without enhancing cyanosis. Vaginal delivery is preferred over CD when feasible, because of the lesser hemodynamic stress if adequate analgesia is provided.
2. Tetralogy of Fallot—“blue baby syndrome.” Tetralogy of Fallot (ToF) is the most common cyanotic lesion, accounting for 10% of CHD.
a. Features of ToF include33,43: (see Fig. 25.6)
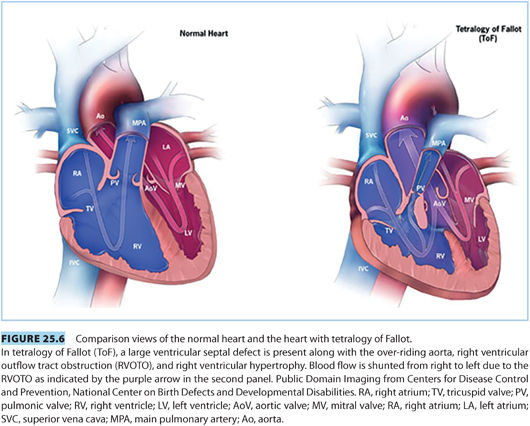
(1) Ventricular septal defect
(2) Pulmonic stenosis/right ventricular outflow tract obstruction
(3) Overriding aorta
(4) Right ventricular hypertrophy
There is great variability in the extent of these defects ranging from small ventricular septal defect (VSD) and overriding aorta with minimal pulmonary stenosis to severe pulmonary stenosis and large VSD. Unrepaired, only 30% of children will reach the age of 10 years. However, with current surgical repair techniques, there is a 36-year survival of nearly 86%.36
b. Pathophysiology. ToF can cause right-to-left shunt and cyanosis, with the degree of shunting proportionate to the severity of the right ventricular outflow tract obstruction (RVOTO) and SVR.39,44
(1) Palliative shunts (e.g., Blalock-Taussig, Waterston, or Potts) were the initial surgical solutions developed. They all involve systemic arterial (i.e., aorta or subclavian artery) to pulmonary artery anastomoses. They provided temporary relief of symptoms, but often had long-term sequelae.36,37,39
(2) Definitive surgical repair involves closure of the VSD and relief of RVOTO using resection and reconstruction with Gore-Tex patch grafting across the RVOTO or conduits to bypass the RVOTO.44
(3) Common reasons for reoperation following definitive repair include residual VSD or recurrence of the VSD (10% to 20%), residual RVOTO or stenosis (10%) leading to right heart failure,39 and rarely RV failure caused by pulmonic insufficiency from the RVOTO patch.
(4) Additional management concerns include a higher risk of sudden cardiac death compared with age-matched controls, elevated risk of dysrhythmias (especially atrial fibrillation), right bundle branch block, pulmonary regurgitation, and right ventricular aneurysms.
c. Peripartum considerations. ToF patients with definitive surgical repair tolerate pregnancy well and can be managed as a typical obstetric patient.
(1) Patients with mild ToF may present unrepaired for management during the peripartum period. A review of Mayo Clinic data looking at 43 ToF patients found 8 with unrepaired ToF who delivered successfully. This study also confirmed results of two previous case series indicating that patients with repaired ToF are not at increased maternal risk with pregnancy.42 The authors found that the presence of underlying cardiac dysfunction or residual cardiac defects after repair were associated with adverse cardiac events during pregnancy.
(2) Echocardiography should be utilized during pregnancy to monitor maternal cardiac function and adaptation to pregnancy (see Fig. 25.7).
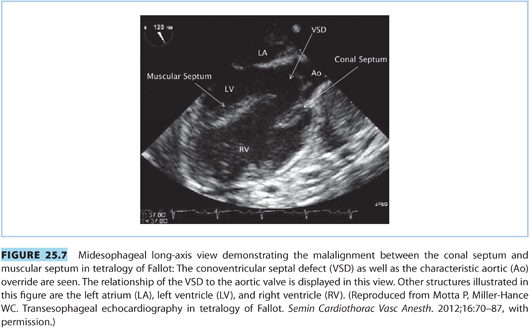
(3) The increased blood volume and decreased SVR can either produce new right-to-left shunting, exacerbate preexisting right-to-left shunting across a patent VSD, and induce cyanosis or heart failure.
d. Anesthetic management2,40,42
(1) Epidural or CSE is an excellent option for pain control early in the first stage of labor, using intrathecal opioid followed by slow epidural titration of dilute bupivacaine. This allows slow equilibration to prevent rapid changes in SVR, preserving the shunt fraction.
(2) Treatment of hypotension should be immediate and most often should be done with phenylephrine. Phenylephrine may cause elevations in PVR, which can be a problem with residual VSD in the absence of RVOTO. In those situations, carefully titrated ephedrine is probably a better choice. In the presence of RVOTO and right-to-left shunting, phenylephrine is preferred because it will increase SVR and decrease shunting.
(3) Patients with pulmonary stenosis or significant pulmonic valvular regurgitation are more likely to develop right heart failure. Avoiding elevated PVR and maintaining high-normal filling pressures are critical in patients with pulmonic valvular regurgitation.40
(4) Cesarean deliveries can be managed with epidural anesthesia using a slow titration of local anesthetic while avoiding large decreases in cardiac preload.
(5) General anesthesia. Choice of induction agents should be tailored to achieve the mentioned hemodynamic goals based on the underlying cardiac function.
(6) If an arterial catheter is placed, patients with Blalock-Taussig shunts will require cannulation in the contralateral arm or in either leg.
CLINICAL PEARLAnesthetic and hemodynamic goals in ToF:
• Avoid changes (especially decreases) in SVR to prevent altering existing shunt.
• Avoid increases in PVR by preventing hypoxia, hypercarbia, acidosis, and providing supplemental oxygen (see Table 25.3).
• Maintain normal to elevated cardiac filling pressures, especially in patients with right ventricular impairment. Avoid aortocaval compression.
• Continuous electrocardiogram (ECG) monitoring is advisable due to high incidence of both atrial and ventricular dysrhythmias.
• Tachycardia and increases in myocardial contractility should be avoided in situations where there is residual RVOTO because this may exacerbate the obstruction and cause right-to-left shunting.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








