Andrea Efre
Cardiac Arrhythmias
Cardiac arrhythmias are electrical abnormalities of the cardiac conduction system that can vary in severity from trivial to life-threatening. These may occur in the presence or absence of structural heart defects or cardiac disease. Arrhythmias may be divided into categories by rate or location. Rate classifies the arrhythmia by the speed of the heart rate: tachyarrhythmia (>100 beats per minute) and bradyarrhythmia (<60 beats per minute). Location identifies where the arrhythmia originates: ventricular (originates from the ventricles) and supraventricular (originates from above the ventricles). Arrhythmias may arise from conductive tissue anywhere within the atria, atrioventricular (AV) junction, or the ventricles. However, symptoms are more closely related to the ventricular rate and to the severity of underlying heart disease than to the origin of the arrhythmia. Cardiac arrhythmias may cause minor symptoms such as palpitations or dizziness but may also predispose to the development of lethal conditions such as stroke, embolism, or sudden cardiac death.1
Definition and Epidemiology
Tachyarrhythmias
More than half of all cardiac arrhythmias arise from or involve the atria. Atrial fibrillation (AF), atrial flutter, and other supraventricular tachyarrhythmias occur frequently in patients with ST-elevation myocardial infarction (STEMI). They are triggered by excessive sympathetic stimulation, atrial stretch caused by ventricular overload, atrial infarction, pericarditis, electrolyte abnormalities, hypoxia, or underlying lung disease.2 By far the most common supraventricular arrhythmia is AF (estimated 2.3 million Americans), which is often associated with structural heart disease or other co-occurring chronic conditions. It is slightly more common in men, and there is an increased prevalence with age (older than 60 years of age).3,4 AF occurs in 8% to 22% of patients with STEMI, and new-onset AF is associated with shock, heart failure (HF), stroke, and 90-day mortality.2
Ventricular tachyarrhythmias, especially in the presence of serious underlying organic cardiac disease, may predispose the patient to sudden cardiac death and increased mortality rates. Sudden cardiac death claims 300,000 to 400,000 lives annually in the United States, with the majority being older adults; approximately 80% of these deaths are caused by ventricular fibrillation (VF) in the context of ischemic heart disease. Structural cardiovascular anomalies and congenital rhythm abnormalities (such as long QT syndrome or Brugada syndrome) are thought to be the cause of unexplained deaths in the younger population.1
Ventricular arrhythmias such as sustained ventricular tachycardia (VT) and VF are common early after the onset of STEMI and are also the most common cause of out-of-hospital cardiac arrest with STEMI. There are multiple factors in the possible cause of these arrhythmias, which include ongoing ischemia, hemodynamic instability, electrolyte abnormalities, enhanced automaticity, and reentry mechanisms.2 Ventricular arrhythmias such as accelerated idioventricular rhythm may follow myocardial insult and are known as reperfusion arrhythmias; they are related to the restoration of normal myocardial blood flow and may occur after myocardial infarction (MI) or revascularization procedures such as percutaneous coronary intervention.
Bradyarrhythmias
Bradyarrhythmias may result from abnormalities in conduction between the sinoatrial (SA) node and atrium, within the AV node, or in the intraventricular conduction pathways. The rhythm of sinus bradycardia may be noted as an incidental finding and considered normal in highly trained athletes. The presence of symptoms with sinus bradycardia should lead to further investigation and a possible treatment plan.
Bradyarrhythmia may result from intrinsic disease of the sinus node or AV conduction system, but the cause of intermittent bradyarrhythmia can be difficult to determine.5 Idiopathic fibrosis is a major cause of AV block, particularly in older adults.4 Other causes may include hypothyroidism, advanced liver disease, hypothermia, or severe hypoxia or medications such as calcium channel blockers or beta blockers. Other causes of disruption in normal conduction may include coronary spasm, myocarditis, rheumatic fever, mononucleosis, Lyme disease, sarcoidosis, amyloidosis, and neoplasms.
Bundle branch block (BBB) may be intermittent or chronic, and symptomatic or asymptomatic. Possible causes include structural heart disease, congenital conditions, cardiac disease, and coronary artery disease. Right bundle branch block (RBBB) is associated with right ventricular hypertrophy, ischemic heart disease, pulmonary embolus, atrial septal defect, rheumatic heart disease, myocarditis, cardiomyopathy, and Brugada syndrome. Left bundle branch block (LBBB) is associated with ischemia, MI, aortic stenosis or regurgitation, dilated cardiomyopathy, and Lyme disease. The rhythm disturbances of BBB do not always require treatment, but it is imperative to determine and manage the underlying cause as well as to treat any associated symptoms.
Pathophysiology
The functional components of the cardiac conduction system subdivide into (1) impulse-generating tissue (SA and AV nodes) and (2) impulse-propagating tissue (e.g., His-Purkinje system). The SA node is the impulse-generating node with the highest intrinsic firing rate. It has complex dimensional tissue composed of ion channel and gap junction expression profiles, which have different action potential characteristics and conduction properties.6 Rhythmic release of calcium contributes to the SA nodal diastolic depolarization. The local intracellular calcium elevations drive the sodium-calcium exchange current to substitute intracellular calcium for extracellular sodium, producing positive-charge results in membrane depolarization.6
The SA node is the natural pacemaker of the normal heart; if stimulated, suppressed, or blocked, it will induce tachyarrhythmias or bradyarrhythmias. The pacemaker function may then be assumed by “escape” foci in the atrial tissue, the AV node, the bundle of His, the Purkinje fibers, or the ventricular myocardium. The intrinsic rates of each part of the conductive system may be influenced (increased, decreased, or blocked) by factors such as cardiac disease, ischemia, medications, electrolytes, or changes in the endocrine system. Impaired conduction through the cardiac conduction system may be related to altered action potential (usually a result of ion channel defects) or defective coupling between cardiomyocytes (e.g., AV nodal block or BBB).6
Cardiac arrhythmias are a result of abnormal impulse formation or conduction and can be categorized into one or more of three mechanisms: (1) abnormal automaticity, (2) triggered activity, or (3) reentry.
• Abnormal automaticity (enhanced or suppressed): Automaticity is a natural property of all myocytes, which may be suppressed or enhanced by factors such as electrolyte imbalance, medications, hypoxia, ischemic heart disease, scarring, or increased age, and can result in atrial or ventricular tachyarrhythmias.7 Abnormal or enhanced automaticity is a deficit in the ability of the cardiac cells to depolarize spontaneously. Enhanced automaticity results in increased conduction of impulses and results in tachycardia (e.g., sinus tachycardia). Abnormal automaticity may lead to irregularity of the impulse conduction, causing erratic or ectopic rhythms of the atria or ventricles. Suppressed automaticity decreases conduction of the SA node and can result in sinus node dysfunction or sick sinus syndrome.
• Triggered activity: Triggered activity usually occurs after an early or delayed depolarization that precipitates multiple depolarizations and causes ventricular arrhythmias. They may be induced by electrolyte imbalances or medications, as in antiarrhythmic or digoxin toxicity.7 An example of a triggered arrhythmia is torsades de pointes.
• Reentry: Reentry arrhythmias require a circular movement of the impulse across the myocardium. Most tachyarrhythmias are thought to be caused by a reentry mechanism and include bidirectional conduction and unidirectional block.7 They may start and terminate suddenly and are often paroxysmal. If a large area is involved, it is known as a macro-reentry arrhythmia and occurs through concealed accessory pathways; the best example is Wolff-Parkinson-White (WPW) syndrome, but macro-reentry arrhythmias can also cause AF and atrial flutter. Micro-reentry arrhythmias affect a small area; examples include VT or VF after MI. An example of a reentry arrhythmia is AV nodal reentry tachyarrhythmia.
The pathophysiology of arrhythmias is additionally defined in the differential diagnosis section to include the expected electrocardiographic changes.
Clinical Presentation
Tachyarrhythmias
Tachyarrhythmias may be entirely asymptomatic or may cause symptoms that affect the patient’s activities of daily living. When symptoms occur they are in large part related to the ventricular rate, extent of underlying heart disease, ventricular function, and associated precipitating factors. Palpitations are the most common symptom caused by tachyarrhythmias. In patients with paroxysmal attacks, palpitations start and terminate abruptly and are usually rapid but regular. In patients with AF the palpitations are typically irregular and tend to be more sustained. Extrasystoles may also cause palpitations or an awareness of isolated extra beats; and the pause that follows may be symptomatic. Other causes of palpitations include thyrotoxicosis, hypovolemia, regurgitant valvular disease, anemia, hypoglycemia, pheochromocytoma, fever, anxiety, symptoms of menopause, stimulants such as caffeine, street drugs, and medications. Any history of underlying heart disease or previous rhythm disturbance and its treatment is relevant, as are the family history and evaluation of coronary risk factors. Pertinent history also includes inquiring about the use of alcohol, tobacco, caffeine, sympathomimetics (commonly found in over-the-counter cold medicines or diet aids), and prescription medication use (e.g., theophylline or thyroid supplements). When interviewing the patient with tachyarrhythmias and/or palpitations, it is prudent to ask about the use of street drugs, especially those known to be stimulants, such as cocaine, methamphetamines, synthetic cannabinoids, and synthetic cathinones (bath salts).
Tachyarrhythmias tend to shorten diastole, and ventricular filling may become compromised, causing a drop in blood pressure, cardiac output, and coronary perfusion. Irregularity in the ventricular rate control, loss of coordinated atrial contraction (e.g., AF), or beat-to-beat variability in ventricular filling causes symptoms that may include lightheadedness, dizziness, syncope, dyspnea, or fatigue, with fatigue being the most common presenting symptom of AF.3 A serious tachyarrhythmia may result in hemodynamic decompensation, causing hypotension, chest pain, HF, change in level of consciousness, or even sudden cardiac death. It is important to assess both the arrhythmia and its tolerance by the patient to determine the degree of urgency and the appropriate setting for intervention.
Bradyarrhythmias
Bradycardia may cause symptoms or may be asymptomatic (especially in healthy individuals), discovered as an incidental finding on routine electrocardiography. In such cases it is most likely that the needs of the body are being met despite the slow heart rate. Symptoms accompanying bradycardia are largely dependent on the ventricular rate relative to metabolic demand and on the presence of underlying cardiac disease. Those with limited cardiac reserve are less tolerant of a slow rate than those with normal hearts. The American Heart Association recognizes two types of bradycardia: absolute and relative. Absolute bradycardia refers to any heart rate below 60 beats per minute. Relative bradycardia refers to a heart rate that is too slow to maintain normal blood pressure or cardiac output even if the rate is greater than 60 beats per minute.8
Symptomatic bradycardia is thought to be directly responsible for the development of frank syncope or near-syncope, transient dizziness, lightheadedness, or confusion resulting from decreased cerebral blood flow attributable to the slow ventricular rate.5,8 Subtle symptoms of irritability, lassitude, inability to concentrate, apathy, or forgetfulness are common symptoms of bradyarrhythmia, and palpitations may be the presenting complaint when the bradyarrhythmia is a manifestation of sick sinus syndrome. Other symptoms may occur at rest or with exertion in persistent bradyarrhythmias, and include fatigue, reduced exercise capacity, or symptoms of HF.5,8 In addition, lethargy; weight gain; changes to the skin, hair, or nails; constipation; or eyelid edema may be subjective findings of hypothyroidism, which may be the cause of a bradyarrhythmia.
Relevant aspects of the history include a careful review of all medications and the identification of any underlying cardiac disease. It is important to discern whether the symptoms occur at rest or with exertion and if there are outstanding, aggravating, or alleviating factors. A vagal mechanism for bradycardia may be implicated—for instance, if the symptoms occur only with straining, such as with vomiting or moving the bowels.
Physical Examination
The initial examination should include evaluation of blood pressure, pulse, temperature, mental status, evidence of diaphoresis, respiratory effort, and manifestations of anxiety. Alterations in rate and pulse volume and irregularity may accompany ventricular ectopic beats, depending on the timing and force of ventricular contractions, which may be observed by labile blood pressure. Orthostatic vital signs are helpful to exclude orthostatic hypotension as a cause of syncope, dehydration, or hypovolemia, which could be the cause of reflex tachycardia (requiring prompt intervention). The patient’s hydration status also includes examination of skin turgor and status of mucous membranes.
Assessment of the neck should include inspection, observing the neck veins for jugular venous distention (a sign of HF), and for the presence of a goiter (suggesting thyroid disorder). Assessment of the neck veins may provide information about atrial activity. The a wave (atrial contraction) reflects atrial pressure and occurs just before S1 and the carotid pulse. The v wave (venous filling) occurs just before or coincides with S2.9 Absence of the a wave suggests AF as atrial systole is lost. A more prominent a wave than v wave may be observed with 2:1 AV block. Cannon a waves, or forceful, irregular expansions in the jugular pulse, may occur with AV dissociation as the atria contract against closed AV valves, causing a reflux of blood to the jugular veins.10
The carotid pulses are palpated for amplitude, contour, timing, and presence of thrills. Auscultation of the carotid arteries should be performed initially with the diaphragm of the stethoscope to detect the higher frequency of the arterial bruits, then with the bell to detect the low-pitched sounds of higher-grade stenosis.9 The presence of a bruit would suggest atherosclerosis and contraindicates carotid massage as a diagnostic or treatment option.
The chest is inspected and palpated for parasternal lifts, heaves, and thrills. Palpation of the point of maximum impulse (PMI) and percussion of the left side of the chest will establish the size and location of the heart. Enlargement of the cardiac silhouette may suggest ventricular hypertrophy or cardiomyopathies, which are triggers for some arrhythmias. Auscultation of the heart sounds for regularity, rate, murmurs, clicks, or the presence of extra heart sounds is essential. An accentuated S1 may be heard in some tachyarrhythmias, and a diminished S1 may be found in AV nodal blocks. A varying S1 may be a sign of complete heart block or AF as the mitral valve is in varying positions before ventricular contraction. The splitting of S2 may be heard in patients with premature ventricular contractions (PVCs) or RBBB; and paradoxical splitting of S2 may be related to LBBB.9 An S3 is a significant finding of increased ventricular filling and can be caused by fluid overload, HF, or decreased myocardial contractility, which may be end points of an arrhythmia. Note that S3 may be a normal finding in a child, young adult, or pregnant female, who may also have the presence of a cardiac arrhythmia. The presence of an S4 is pathologic and is caused by resistance in ventricular filling. It may be associated with AV nodal conduction delays.
The presence of an S3 and/or S4 in an athlete should be investigated because it may be a sign of athletic heart syndrome. Electrocardiographic findings may include sinus bradycardia, AV nodal block, or RBBB, and there may be lateral displacement of the PMI owing to the increased heart size. These patients should be referred to a cardiologist for further evaluation because the differentiation of benign findings from the ominous possibility of sudden cardiac death can be difficult to determine.
A heart murmur is most often associated with underlying valve disorders; however, a benign systolic ejection murmur may accompany a tachycardia with or without valvular disease. Absence of a murmur is not necessarily a significant finding because rapid rates can often make accurate auscultation difficult. The patient should always be reexamined after the heart rate is controlled. An S3 sound is a significant finding, as are jugular venous distention (JVD) and peripheral edema—signs of fluid overload—and may warn of impending HF or be an indication that the rhythm is poorly tolerated by the patient.
As part of the complete assessment, the examiner auscultates the lungs for rales, wheezes, or rhonchi. Other important findings include exophthalmos, an enlarged or nodular thyroid gland, or skin, nail, and hair changes commonly associated with hyperthyroidism or hypothyroidism. When the presenting complaint is syncope, near-syncope, dizziness, confusion, or altered level of consciousness, a neurologic examination should be performed to explore the possibility of noncardiac causes.
If there is a positive history, or suspected use, of street drugs, physical manifestations will depend on the type of drug and route used. The examiner should be observant for signs of use: needle markings (e.g., intravenous use), burns to the mouth or fingers (e.g., from pipe use), sores to the nasal area, epistaxis or increased rhinorrhea (e.g., insufflation), or skin or gum abscesses or rotten teeth noted from chemically manufactured drugs or methamphetamine.
Initial Diagnostics
12-Lead Electrocardiogram
The 12-lead electrocardiogram (ECG) is indicated for initial evaluation of a suspected arrhythmia. This diagnostic tool has the notable limitation of providing only a 12-second view of the heart’s electrical activity. Although sustained rhythms may easily be captured, paroxysmal rhythms may be elusive. However, even when the rate and rhythm are normal, the resting ECG may yield valuable information about the cause of the arrhythmia. The first priority is to identify ST- or T-wave changes that indicate MI or myocardial ischemia. Other significant findings are ventricular hypertrophy, effects of medication toxicity, and electrolyte imbalance, such as peaked T waves noted in hyperkalemia.
Indications of conduction abnormalities may also be present and include the widened QRS complex of intraventricular conduction delay, the shortened PR interval that accompanies preexcitation syndromes such as WPW syndrome, and the prolonged QT interval that may accompany idiopathic long QT syndrome or drug effects.4,10 When abnormal rhythms are captured on the 12-lead ECG, it is prudent to record a rhythm strip by allowing the tracing to continue for several minutes to fully evaluate the rhythm. With tachyarrhythmias, minor depressions of the ST segment and inversion of the T wave could be rate related rather than indications of coronary disease.10 However, these changes should be reevaluated once the rate is controlled. Arrhythmias can be a sign of underlying acute coronary syndrome (ACS), and the 12-lead ECG should be interpreted carefully.
Laboratory (Serum Testing)
Based on presenting symptoms, history, and clinical findings, laboratory testing may assist in determining the underlying cause of the arrhythmia. These tests might include a complete blood count (CBC) to determine the presence of anemia or infection; and serum electrolyte values including potassium, calcium, and magnesium to evaluate disturbances such as hypokalemia, hyperkalemia, hypocalcemia, hypercalcemia, or hypomagnesemia. A blood glucose measurement is helpful if hypoglycemia is suspected, and blood urea nitrogen (BUN) and creatinine levels are beneficial in determining fluid volume status. A thyroid-stimulating hormone (TSH) level should be drawn if hyperthyroidism or hypothyroidism is suspected. Measuring a toxicity level may be useful for patients being treated with medications that might cause arrhythmia (e.g., digoxin). Toxicology screening for stimulants such as cocaine or amphetamines may beneficial. Cardiac biomarkers should be measured if ACS or coronary ischemia is suspected as the cause of the arrhythmia (evaluate coronary risk factors for questionable ACS).
Echocardiography
Two-dimensional transthoracic echocardiography with Doppler should be performed during the initial workup of all arrhythmia patients to determine left atrial and left ventricular size, systolic function, and underlying structural heart disease. This is useful in guiding decisions for antiarrhythmic and antithrombotic therapy, particularly with regard to the patient with AF.
Radiologic Imaging
Chest radiography is useful to identify structural disease or the presence of HF or pneumonia.
Stress Testing
Stress tests are an example of provocative testing and can be performed by exercise (usually on a treadmill) or pharmacologically. Causes of exercise-induced arrhythmias may include ischemia, increased sympathetic activity, congenital conduction disturbances, and medications. It is important to verify the absence of coronary ischemia before initiating antiarrhythmic agents. Nuclear images of the myocardium at baseline and after exercise show disruption of myocardial blood flow from ischemia. When the history suggests symptoms such as palpitations, exercise stress testing can be used to provoke electrocardiographic changes or arrhythmias, which may remain unidentified at rest. This is especially useful if the symptoms occur or are worsened with activity. Exercise testing can also be useful to evaluate the adequacy of rate control in AF.3
Other Diagnostics
Holter Monitor
Continuous ambulatory electrocardiographic rhythm monitoring such as with a Holter monitor is a useful option for evaluation of a suspected arrhythmia, especially when symptoms are inconsistent or paroxysmal in nature. Ambulatory monitoring may also be useful in the definitive correlation of bradyarrhythmias when evaluating the need for a pacemaker. Use of this portable device allows continuous recording of the heart’s activity during a 24- to 48-hour period. The patient keeps a diary of activities and symptoms that can later be correlated with the tracing. This is worn while patients go about their usual activities. Ventricular arrhythmias such as PVCs, ventricular couplets, and nonsustained VT may have no similarity among the days, making it unlikely that a single Holter recording for 24 hours may capture this phenomenon, suggesting that additional recordings may be necessary.11
Event Monitor and Loop Recorder
For the patient with infrequent symptoms, intermittent ambulatory electrocardiography (event recording) may be more appropriate because these devices can be worn for a long time. There are two types of these recorders. One type is worn externally; this recorder remains dormant until it is activated by the user at the onset of symptoms.4 Patient-activated electrocardiographic event recorders can help assess the relation to symptoms, whereas auto-triggered event recorders may detect asymptomatic episodes. These technologies may also provide valuable information to guide drug dosage for rate control or rhythm management.3
The second type is an implantable loop recorder for long-term monitoring, used for diagnosis in patients with recurrent unexplained episodes of palpitations or syncope. The implanted device records the ECG during activity or symptoms or can record when it is activated by the patient. The device has an automatic recording triggered by arrhythmia and has an additional feature that allows the patient to activate the recording. New devices may offer remote transmission of the data back to the cardiologist. Implantable loop recorder monitoring is useful in detecting arrhythmias and guiding treatment plans,. It may be used to evaluate for lethal bradycardias and sudden cardiac death in hemodialysis patients.12 The implantable loop recorder may stay in place for months or several years; battery life tends to last 24 months or more. It can provide additional diagnostic value in patients with syncope or nonsyncopal, real or apparent, transient loss of consciousness.13,14
Tilt-Table Test
Provocative testing in the form of a tilt-table test is used to evaluate syncope. The patient is observed as the table is angled in varying degrees. Symptoms and hemodynamic status are monitored to see if syncope is related to a vasodepressor or a cardiac or neurologic reason. Arrhythmias, usually bradyarrhythmias, may be elicited with position change and induce syncopal symptoms. An example is malignant vasovagal syndrome, which is evidenced by exaggerated vagal response to emotional or painful stimuli. Tilt-table tests are performed in a monitored setting by a specialist, usually a cardiologist or an electrophysiology (EP) cardiologist, or a neurologist if syncope is suspected to be non-cardiac.
Electrophysiologic Studies
Rhythms that put the patient at high risk for adverse events warrant referral to a specialist for electrophysiologic studies to properly identify and treat the problematic rhythm. The arrhythmias are often tachycardic in nature and may include very rapid supraventricular tachycardia (SVT), WPW syndrome, complex ventricular ectopy, and VT. EP studies may also be indicated for investigation of palpitations and syncope when noninvasive techniques have failed to definitively identify the problem.4 If congenital heart defects are suspected to be the source of an arrhythmia, electrophysiologic studies may be useful in evaluating if the arrhythmia is related to extra electrical pathways (known as accessory pathways). The procedure will evaluate the origin and specific location of extra pathways and (if reachable and appropriate) may lead to ablation of the offending source cells.
Transesophageal Echocardiography
Transesophageal echocardiography (TEE) is performed by a specialist physician and is used to determine the presence or absence of left atrial thrombus before consideration of cardioversion.4 For patients with asymptomatic WPW syndrome, the TEE procedure can be very helpful in determining their risk for sudden cardiac death.15
Carotid Sinus Massage and Valsalva Maneuvers
Valsalva maneuvers and carotid sinus massage may be used as a diagnostic test to induce bradycardia. Transient AV block results in the slowing of the ventricular response, enabling identification of the underlying rhythm. These techniques may terminate rhythms for which the AV node is part of the reentry circuit, such as in atrioventricular nodal reentry tachycardia (AVNRT), and are therefore used as therapeutic treatments. The provider should evaluate atherosclerotic risk factors, assess for carotid bruits, and ensure correct technique is used before performing carotid massage. Carotid sinus massage during simultaneous recording of the ECG is useful to provoke symptomatic bradycardia in carotid sinus syndrome, a disorder in which bradycardia occurs in response to carotid sinus hypersensitivity. Carotid sinus massage is usually performed by a physician specialist in a monitored setting (e.g., emergency provider, cardiologist or EP cardiologist).
Differential Diagnosis of Tachyarrhythmias
Narrow Complex Tachycardia
Narrow complex tachycardia includes rhythms with a rate over 100 beats per minute and QRS duration of 0.12 second or less. The rhythms in the following paragraphs are included in this group and are described as they appear on the ECG.
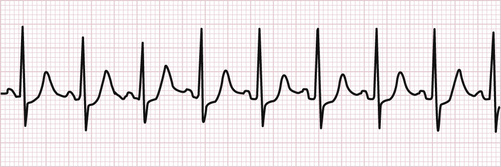
Sinus Tachycardia.
In sinus tachycardia, there is a P wave preceding each QRS complex in a consistent 1:1 relationship. The rhythm is regular, the P waves are identical, the QRS complexes are normal and narrow, and the PR and QRS intervals are within normal ranges. The rate is above 100 beats per minute (Fig. 118-1).