UNIT XII: REPRODUCTIVE CONDITIONS Una T. Hopkins, DNP, RN, FNP-BC The primary care provider (PCP) is the first line of proactive screening for women experiencing breast symptoms. The PCP can be an asset in educating a female patient about her individual risk and what screening program she should follow to ensure early detection of more serious breast conditions. Diseases of the breast are common clinical problems that are frequently seen in patients in primary care practice. The most important aspect of managing breast problems is the early recognition of breast cancer, which is the most commonly diagnosed cancer in women (Breast Cancer Statistics, 2014). The annual mortality rate for breast cancer has remained stable over the last 10 years due to continuous use of proactive screening, early diagnosis, treatment improvements, and advancements (American Cancer Society [ACS], 2014). As a body of evidence for screening methodologies becomes available, it has created controversy and discomfort in the screening community of radiologists, medical oncologists, gynecologists, and PCPs. Together with the patient, the PCP is the crucial first link in the effort toward prevention and early detection of breast cancer. PCPs must become adept at risk assessment and early diagnosis of breast cancer. All women should consider their individual risks for breast cancer and should be informed about the risks and benefits of screening. Until puberty, the female breast is a rudimentary structure consisting of only a few ducts without acini. Under the influence of estrogens, progesterone, and the pituitary hormones, ductal tissue begins to grow, bud, and form acini at puberty. Glandular tissue separates into 12 to 20 lobes, or segments, extending from the nipple in a radial fashion. The ducts from each segment empty into a single lactiferous sinus that terminates at the nipple. The glandular tissues of the breast undergo cyclical changes in response to the hormone fluctuations during the menstrual cycle. At the start of the cycle, the ductal cells proliferate, there is an increase in interstitial fluid, and a lymphocytic infiltration is seen. This reaches a maximum just before the menses, at which time the ducts shrink and the ductal epithelium is shed. The cycle then repeats itself. These changes may lead to subsequent dilatation or hyperplasia of the ducts, hypertrophy of the surrounding connective tissue, and many benign conditions loosely referred to as fibrocystic changes. During pregnancy, the breast lobules undergo maximal proliferation, and alveoli form. This proliferation is stimulated by the placental hormones. After delivery, the withdrawal of the placental hormones, along with prolactin secretion by the pituitary gland, stimulates lactation. Milk is produced by shedding of the alveolar cells. After the completion of lactation, the breast glandular tissue partially involutes. At menopause, there is further involution of the glandular tissue of the breast, with loss of the lobular outlines. Fibroglandular tissue is replaced by fatty tissue. Common abnormalities of breast development include accessory nipples and ectopic glandular tissue. Ectopic nipples may occur anywhere along the “milk line,” which extends from the axillae, through the nipple and down to the inguinal ligament. Accessory breast tissue (polymastia) is most often seen in the axilla; accessory nipples (polythelia) are most often seen in the midclavicular line below the normal breast. The lymphatic drainage of the breast is predominantly toward the ipsilateral axillary lymph nodes. Lymphatic drainage in the medial aspect of the breast may also proceed toward the internal mammary nodes or the interpectoral nodes. Lymphatic spread of breast carcinoma cells may lead to enlargement of the axillary lymph nodes, which may be apparent on physical examination. Breast lesions may be classified into four broad histopathologic categories: 1. Benign, nonproliferative lesions carry no increased risk of subsequent development of malignancy. Benign lesions include: a. Cystic hyperplasia (ibrocystic disease) b. Duct ectasia c. Galactoceles e. Fat necrosis f. Fibroadenoma g. Hamartoma h. Papilloma i. Inflammatory lesions 2. Benign proliferative lesions include: a. Ductal hyperplasia without atypia, which carries no significant, increased risk for subsequent malignancy. b. Atypical ductal hyperplasia, which carries an increased risk of subsequent malignancy. c. Atypical lobular hyperplasia, which also carries an increased risk of subsequent malignancy. d. Sclerosing adenosis, which has no significant link to subsequent malignancy. e. In situ carcinomas are malignant-appearing cells that lack evidence of invasion through the basement membrane. These include: i. Lobular carcinoma in situ (LCIS): It is a sometimes misleading nomenclature as it is not a malignant state but should be considered as an increased risk factor for the subsequent cancer development in either breast. ii. Ductal carcinoma in situ (DCIS), considered a direct precursor to invasive ductal carcinomas. 3. Invasive carcinomas are malignant cells that demonstrate invasion of the basement membrane. These lesions have the potential for metastatic spread. a. Invasive ductal carcinoma (80% of invasive carcinomas). b. Invasive lobular carcinoma (15%) c Medullary carcinoma, tubular carcinoma, mucinous carcinoma, and papillary carcinoma and cribriform, and inflammatory carcinoma (5%). 4. Other malignancies include: a. Paget’s disease of the nipple, Phylloides tumors (20%–30% have malignant features) b. Soft-tissue sarcomas, lymphomas, and metastatic tumors. The incidence of breast cancer steadily increases with age (Cancer Facts and Figures, 2014). Breast cancer is rare in patients under 20 years of age, but its incidence begins to rise rapidly during the childbearing years. According to the National Cancer Institute (NCI) in 2013, there were approximately 232,000 cases of breast cancer discovered in the United States with an annual mortality rate just under 40,000. Women’s risk for breast cancer increases in the fifth decade of life. The rate of breast cancer continues to increase after menopause but at a slower rate. Women in the United States currently have a lifetime risk of developing breast cancer of approximately 12% and a lifetime risk of dying of breast cancer of approximately 2.8% (Cancer Facts and Figures, 2014). The incidence of breast cancer in men is approximately 1% the rate of breast cancer in women. According to the NCI there were 2,240 new cases of male breast cancers diagnosed in 2013 in the United States. Male breast cancers typically present as palpable masses, with a pattern of spread and prognosis similar to those in women (Cancer Facts and Figures, 2014). The incidence of breast cancer is higher in the United States and Western Europe than in other parts of the world. There are several known risk factors for breast cancer. Some risk factors are modifiable and include diet, exercise, and alcohol intake while others such as genetic mutations and age are not. Risk Factors While the causes of breast cancer are not fully understood and are likely to be multifactorial, several of the risk factors known to increase a woman’s risk for breast cancer include: African American women have the highest risk of development of an aggressive tumor before the age of 40 years and the highest mortality rates. The incidence and mortality of African American woman is disproportionate to the Caucasian population (Cancer Facts and Figures, 2014). This increased risk was initially shown to be independent of socioeconomic status by Newman et al. (2006). African American women have a disproportionately high rate of diagnosis in the less than 45 years age category; in contrast to the statistics for Caucasian women where the risk is increased in the fifth decade of life (Morris et al., 2007; Newman et al., 2006). African American women are often diagnosed with a later-stage and higher-grade more aggressive tumors (Morris et al., 2007). Current CDC statistics (2014) continue to show that Black women have the highest breast cancer death rates of all racial and ethnic groups and are 40% more likely to die of breast cancer than White women. The differences seen in the biology of tumors in those women who are of different ethnic and racial backgrounds should be taken into account when deciding upon the appropriate individualized screening plan for the patient. These facts add additional information to the provider when interpreting the guidelines for this specific population of women. Women of African American background have a mortality rate about 10% higher than White women, even when matched for stage. The incidence of breast cancer shows consistency across all socioeconomic groups. The reasons for this are not clear, but may be related to reproductive, dietary, or environmental factors or to the pathology of the tumors (Cancer Facts and Figures, 2014). Evaluation of Breast Problems In the process of screening for and evaluating patients for breast cancer, the PCP is likely to encounter a number of other common breast complaints. What follows are a series of algorithms designed to help the provider understand the evaluation of nipple discharge and the palpable mass and delineate when further evaluation for cancer is appropriate (Figures 63.1 and 63.2). Beyond these are a lgorithms designed to help the clinician understand the process of managing abnormalities on mammograms: microcalcifications and nonpalpable masses. According to NCCN guidelines mammographic evaluation of an abnormality should be followed up based upon the Breast Imaging-Reporting and Data System (BI-RADS) category the abnormality falls into. Any BI-RADS 4, 5, or 6 should proceed to the appropriate diagnostic workup for biopsy and further imaging. The challenge for the PCP is the mammogram that is under category BI-RADS 3, which encourages continued screening at an increased frequency. If an abnormality is stable after 2 years of increased frequency in screening, there can be a return to annual screening (NCCN, 2014). The discussion of risk factors associated with breast cancer should be included in the decision to initiate screening. Decisions about when to initiate screening and how that screening should be carried out are important and should be based on the best evidence available. Unfortunately at the time this is being written, there is no clear consensus on the best screening methods and the effectiveness of existing screening regimens. Conflicting guidelines and expert opinions give providers information and data with which to facilitate a conversation between provider and patient. Together this team (patient and provider) can assess risk and decide best screening methods and intervals. In 2009, the United States Preventive Services Task Force (USPSTF) completed a review and grading of available evidence on mammography, ultrasound, and MRI that provided recommendations for the initiation of screening and which technology to utilize. Summary of USPSTF Recommendations 1. The USPSTF recommends biennial screening mammography for women aged 50 to 74 years. Grade: B recommendation. 2. The decision to start regular, biennial screening mammography before the age of 50 years should be an individual one and take patient context into account, including the patient’s values regarding specific benefits and harms. Grade: C recommendation. 3. The USPSTF concludes that the current evidence is insufficient to assess the additional benefits and harms of screening mammography in women 75 years or older. Grade: I statement. 4. The USPSTF recommends against teaching breast self-examination (BSE) Grade: D recommendation. 5. The USPSTF concludes that the current evidence is insufficient to assess the additional benefits and harms of clinical breast examination (CBE) beyond screening mammography in women 40 years or older. Grade: I statement. 6. The USPSTF concludes that the current evidence is insufficient to assess the additional benefits and harms of either digital mammography or MRI instead of film mammography as screening modalities for breast cancer. Grade: I statement (USPSTF, 2009). Trial results published in 2014 in the British Medical Journal by Miller, Wall, Baines, Sun, To, and Narod further describe the lack of relationship between mammography screening and mortality from breast cancer (Miller et al., 2014). This research has added to the controversy about the role of mammography screening in women. Evidence of the ineffectiveness of mammographic screening has, in turn, been countered by many critics (Kopans, 2013). They point out significant changes and improvement in screening since earlier studies were conducted and the lack of racial and ethnic diversity of the women screened (Kopans, 2013). FIGURE 63.1 BI-RADS, Breast Imaging-Reporting and Data System; NCCN, National Comprehensive Cancer Network. FIGURE 63.2 BI-RADS, Breast Imaging-Reporting and Data System. Women in high-risk groups or those with dense breast tissue should be advised of the additional supplemental screening recommendations of ultrasound and or MRI addressed in detail later in this chapter. In some states, women whose mammograms show BI-RADS 3 or 4 for breast density must be told that they have “dense breasts” in the summary of the mammogram report that is sent to patients (sometimes called the lay summary). The language used is mandated by law, an example of the language you may see on the reports is “Your mammogram shows that your breast tissue is dense. Dense breast tissue is common and is not abnormal. However, dense breast tissue can make it harder to evaluate the results of your mammogram and may also be associated with an increased risk of breast cancer.” This information about the results of your mammogram is given to you to raise your awareness and to inform your conversations with your health care provider. Together, you can decide which screening options are right for you” (ACS, 2014). This gives the primary care clinician an opportunity to further discuss screening modalities with the patient. Mammography alone will detect only 85% to 90% of breast cancers. Diagnostic workup is largely directed by abnormalities found on examination and imaging and by correlationt to pathologic findings. The PCP must document the following: The provider should screen for the following symptoms: The clinical breast examination should focus on: Screening mammography is a routine component of health maintenance after assessments of risk and the development of a personalized screening program. Screening mammograms should include: It is interesting to note that in STORM, a prospective population-based trial comparing sequential screen reading of two-dimensional (2D) mammography alone and integrated 2D/three-dimensional (3D) mammography, there was a statistically significant improvement in diagnosis of breast cancers with the use of 2D/3D mammography. This and other improvements in radiographic tools may lead to changes in future recommendations for screening (Bernardi et al., 2014). Mammogram results are reported using a system called BI-RADS (ACR, 2014). This system of reporting is published and trademarked by the ACR. The categories are listed below: Category 1: Negative Category 2: Benign (noncancerous) finding Category 3: Probably benign finding—follow-up in a short time frame is suggested Category 4: Suspicious abnormality—biopsy should be considered Category 5: Highly suggestive of malignancy—appropriate action should be taken Category 6: Known biopsy-proven malignancy—appropriate action should be taken
CHAPTER 63
Breast Cancer: Screening, Diagnosis, and Treatment
 ANATOMY, PHYSIOLOGY, AND PATHOLOGY
ANATOMY, PHYSIOLOGY, AND PATHOLOGY
 EPIDEMIOLOGY
EPIDEMIOLOGY
 A family history of breast or ovarian cancer, particularly if one or more first- or second-degree relatives are affected. Specific genetic abnormalities, including mutations of the BRCA1 and BRCA2 genes have recently been described. If present, such genetic abnormalities have been found to confer a high risk (50%–90%) that the patient will develop breast cancer. Other genetic syndromes predisposing patients to breast cancer include Li-Fraumeni (p53 gene mutation), Cowden’s, and ataxia-telangiectasia (National Comprehensive Cancer Network [NCCN] Guidelines Version 1, 2014a). Family members with premeno-pausal breast cancer or male relatives with breast cancer also indicate greater risk.
A family history of breast or ovarian cancer, particularly if one or more first- or second-degree relatives are affected. Specific genetic abnormalities, including mutations of the BRCA1 and BRCA2 genes have recently been described. If present, such genetic abnormalities have been found to confer a high risk (50%–90%) that the patient will develop breast cancer. Other genetic syndromes predisposing patients to breast cancer include Li-Fraumeni (p53 gene mutation), Cowden’s, and ataxia-telangiectasia (National Comprehensive Cancer Network [NCCN] Guidelines Version 1, 2014a). Family members with premeno-pausal breast cancer or male relatives with breast cancer also indicate greater risk.
 Early onset of menarche: Higher breast cancer risks are seen in patients with onset of menses before the age of 12 years than in those with menarche after the age of 15 years. This is thought to be due to the amount of time for estrogen exposure in a woman’s lifetime (NCCN Guidelines Version 1, 2014b).
Early onset of menarche: Higher breast cancer risks are seen in patients with onset of menses before the age of 12 years than in those with menarche after the age of 15 years. This is thought to be due to the amount of time for estrogen exposure in a woman’s lifetime (NCCN Guidelines Version 1, 2014b).
 Late onset of menopause: Higher breast cancer risks are seen in patients with natural menopause after the age of 55 years than in those with menopause before the age of 45 years (NCCN Guidelines Version 1, 2014b).
Late onset of menopause: Higher breast cancer risks are seen in patients with natural menopause after the age of 55 years than in those with menopause before the age of 45 years (NCCN Guidelines Version 1, 2014b).
 Late age at first pregnancy: Women who have their first pregnancy after the age of 30 years have been seen to have a small but significantly increased risk of developing breast cancer (Cancer Facts and Figures, 2014).
Late age at first pregnancy: Women who have their first pregnancy after the age of 30 years have been seen to have a small but significantly increased risk of developing breast cancer (Cancer Facts and Figures, 2014).
 Dense breast tissue on mammography has been shown to be a significant risk factor and can increase a woman’s risk as much as having two family members with breast cancer (Cancer Facts and Figures, 2014).
Dense breast tissue on mammography has been shown to be a significant risk factor and can increase a woman’s risk as much as having two family members with breast cancer (Cancer Facts and Figures, 2014).
 Postmenopausal obesity
Postmenopausal obesity
 Previous history of radiation to the chest
Previous history of radiation to the chest
 Previous history of ovarian cancer
Previous history of ovarian cancer
![]() CLINICAL WARNING:
CLINICAL WARNING:
 SCREENING
SCREENING
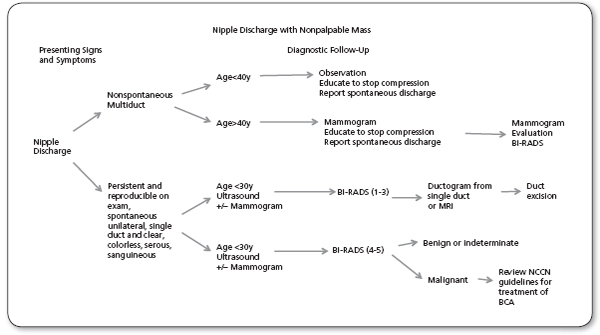
Evaluation of nipple discharge.
Source: Adapted from NCCN, 2014.
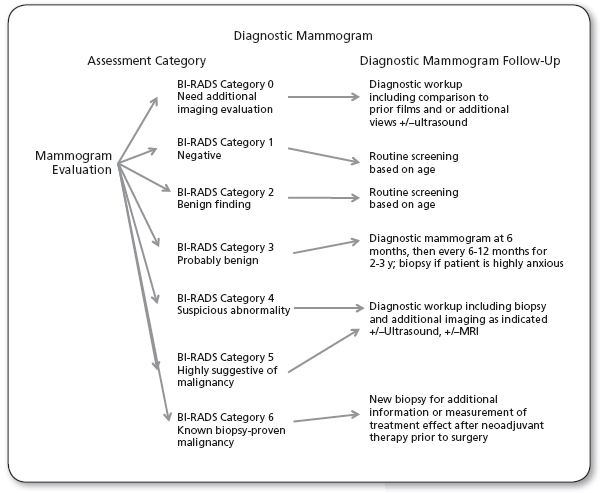
Mammographic evaluation.
Source: Adapted from NCCN, 2014
![]() CLINICAL WARNING:
CLINICAL WARNING:
 DIAGNOSTIC WORKUP
DIAGNOSTIC WORKUP
 HISTORY AND PHYSICAL EXAMINATION
HISTORY AND PHYSICAL EXAMINATION
 Age (pre- or postmenopausal)
Age (pre- or postmenopausal)
 Age of menarche and, if applicable, menopause
Age of menarche and, if applicable, menopause
 Age of first full-term pregnancy
Age of first full-term pregnancy
 Use of hormones
Use of hormones
 Family history of breast and other cancers specifically ovarian or pancreatic
Family history of breast and other cancers specifically ovarian or pancreatic
 Prior breast biopsies
Prior breast biopsies
 Palpable lesion
Palpable lesion
 Skin or nipple changes, asymmetry
Skin or nipple changes, asymmetry
 Nipple discharge color, consistency, in relation to the menstrual cycle
Nipple discharge color, consistency, in relation to the menstrual cycle
 Breast pain
Breast pain
 Inspection:
Inspection:
Skin dimpling, skin redness, nipple retraction, nipple excoriation, or visible masses
 Palpation:
Palpation:
Palpable masses, asymmetric thickening, nipple discharge axillary lymph nodes cervical lymph nodes
 DIAGNOSTIC STUDIES
DIAGNOSTIC STUDIES
 Standard two views of each breast: craniocaudal (CC) and mediolateral oblique (MLO)
Standard two views of each breast: craniocaudal (CC) and mediolateral oblique (MLO)
 Additional diagnostic mammogram views if abnormalities are seen.
Additional diagnostic mammogram views if abnormalities are seen.
 There’s no significant abnormality to report. The breasts look the same (they are symmetrical) with no masses (lumps), distorted structures, or suspicious calcifications. In this case, negative means nothing bad was found.
There’s no significant abnormality to report. The breasts look the same (they are symmetrical) with no masses (lumps), distorted structures, or suspicious calcifications. In this case, negative means nothing bad was found.
 This is also a negative mammogram result (there is no sign of cancer), but the reporting doctor chooses to describe a finding known to be benign, such as benign calcifications, lymph nodes in the breast, or calcified fibroadenomas. This ensures that others who look at the mammogram will not misinterpret the benign finding as suspicious. This finding is recorded in the mammogram report to help when comparing to future mammograms.
This is also a negative mammogram result (there is no sign of cancer), but the reporting doctor chooses to describe a finding known to be benign, such as benign calcifications, lymph nodes in the breast, or calcified fibroadenomas. This ensures that others who look at the mammogram will not misinterpret the benign finding as suspicious. This finding is recorded in the mammogram report to help when comparing to future mammograms.
 The findings in this category have a very high chance (>98%) of being benign (not cancer). The findings are not expected to change over time. But since it’s not proven benign, it’s helpful to see if the area in question does change over time.
The findings in this category have a very high chance (>98%) of being benign (not cancer). The findings are not expected to change over time. But since it’s not proven benign, it’s helpful to see if the area in question does change over time.
 Follow-up with repeat imaging is usually done in 6 months and regularly after that until the finding is known to be stable (usually at least 2 years). This approach helps avoid unnecessary biopsies, but if the area does change over time, it still allows for early diagnosis.
Follow-up with repeat imaging is usually done in 6 months and regularly after that until the finding is known to be stable (usually at least 2 years). This approach helps avoid unnecessary biopsies, but if the area does change over time, it still allows for early diagnosis.
 Findings do not definitely look like cancer but could be cancer. The radiologist is concerned enough to recommend a biopsy. The findings in this category can have a wide range of suspicion levels. For this reason, some doctors divide this category further:
Findings do not definitely look like cancer but could be cancer. The radiologist is concerned enough to recommend a biopsy. The findings in this category can have a wide range of suspicion levels. For this reason, some doctors divide this category further:
 4A: Finding with a low suspicion of being cancer
4A: Finding with a low suspicion of being cancer
 4B: Finding with an intermediate suspicion of being cancer
4B: Finding with an intermediate suspicion of being cancer
 4C: Finding of moderate concern of being cancer, but not as high as Category 5
4C: Finding of moderate concern of being cancer, but not as high as Category 5
 The findings look like cancer and have a high chance (at least 95%) of being cancer. Biopsy is very strongly recommended.
The findings look like cancer and have a high chance (at least 95%) of being cancer. Biopsy is very strongly recommended.
 This category is only used for findings on a mammogram that have already been shown to be cancer by a previous biopsy. Mammograms may be used in this way to see how well the cancer is responding to treatment (ACR, 2014).
This category is only used for findings on a mammogram that have already been shown to be cancer by a previous biopsy. Mammograms may be used in this way to see how well the cancer is responding to treatment (ACR, 2014).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree