
Bradydysrhythmias
David Hartman and David T. Overton
The cardiac conduction system consists of pacemaker cells, conducting cells, and contractile cells. Pacemaker cell possess the capacity to spontaneously depolarize (termed automaticity). The sinoatrial (SA or sinus node) is normally the predominant pacemaker of the heart, is located at the junction of the right atrium and the superior vena cava, and has an intrinsic basal rate of 60 to 100 bpm. The SA node is innervated by both sympathetic and parasympathetic nerves that alter its discharge rate. The arterial blood supply is either from the right coronary artery (55%) or the left circumflex artery (45%).
The atrioventricular (AV) node is located beneath the right atrial endocardium superior to the insertion of the septal leaflet of the tricuspid valve and can also function as a pacemaker, with an intrinsic rate of 45 to 60 bpm. The arterial blood supply of the AV node is from the posterior descending artery of the right coronary artery (90%) or the left circumflex artery (10%). This anatomy becomes clinically relevant in the AV nodal dysrhythmias associated with acute inferior myocardial infarction. The AV node fires in the absence of sinus node impulses, or it may usurp control from the sinus node. Other cells in the bundle branches and Purkinje network can function as pacemakers as well, but their intrinsic rate is quite low, approximately 30 to 40 bpm.
Electrical impulses in the heart travel over a network of conducting cells. Impulses normally originate in the sinus node. Passage through the AV node is relatively slow, accounting for a normal physiologic delay in ventricular depolarization. The AV node blends into the bundle of His, which divides into bundle branches. The bundle branches arborize into a network of Purkinje fibers that transmit impulses to the ventricular myocardial contractile cells. Contractile cells in the atria and the ventricles do not depolarize spontaneously.
The electrocardiographic P wave represents atrial depolarization. The P–R interval (normally 0.12 to 0.20 seconds in the adult) reflects intra-atrial, AV nodal, and His–Purkinje conduction. The QRS complex, representing ventricular depolarization, has a normal duration of 0.04 to 0.10 seconds, and the following T wave represents ventricular repolarization. The U wave is seen in a variety of circumstances such as hypokalemia, although its electrophysiologic basis is uncertain. Two basic pathophysiologic mechanisms underlie the production of both tachydysrhythmias and bradydysrhythmias: (i) disorders of impulse formation and (ii) disorders of impulse conduction. Depressed automaticity may result in bradydysrhythmias, such as sinus bradycardia or sinus arrest. If SA node automaticity is sufficiently depressed, escape rhythms originating elsewhere in the heart may assume control. Depressed impulse conduction may lead to AV or fascicular blocks.
ED EVALUATION AND MANAGEMENT
When approaching patients with bradydysrhythmias, as with any patient, the priorities of airway, breathing, and circulation must be addressed first. The patient should be placed on a cardiac monitor, and an electrocardiogram (ECG) should be obtained expeditiously. The urgency and means of treating bradydysrhythmias depend on how symptomatic the dysrhythmia is, the clinical setting in which the dysrhythmia occurs, the propensity for the dysrhythmia to progress, and concurrent drug therapy. Bradydysrhythmias may produce hypotension, lightheadedness, dizziness, fatigue, mental status changes, angina, congestive heart failure, syncope, or even seizures. The elderly may be more susceptible to symptomatic bradycardia. Prompt therapy using electronic pacing or intravenous medications is required for these symptoms, which are caused by end-organ hypoperfusion. Many dysrhythmias are asymptomatic and require no treatment. A dysrhythmia that occurs during an acute myocardial infarction often mandates a different treatment approach than a dysrhythmia incidentally discovered during a routine physical examination. Electrolyte imbalances and a wide variety of drugs (e.g., digitalis, β-blockers, calcium channel blockers, cocaine, methylprednisolone, fentanyl, and amiodarone) may cause bradydysrhythmias, and each case has its own implications for treatment (1–7).
Sleep apnea may also be a cause of bradycardia (8), as may seizure disorders (9). Propofol has also been implicated in causing acute refractory bradycardia especially at doses >4 mg/kg/h for a prolonged time (10). Many infectious diseases have been reported to cause a relative bradycardia, including typhoid, Legionnaires, malaria, and dengue fever (11). Symptomatic bradycardias are increasingly common with advancing age. General supportive measures such as oxygenation, ventilation, and correction of electrolyte abnormalities are usually the first step. Specific drug therapy or artificial cardiac pacing may also be required.
Among drug therapies, the cornerstone is atropine sulfate, a vagolytic agent that enhances sinus node automaticity and AV nodal conduction. The usual adult dose is 0.5 to 1.0 mg intravenous push every 3 to 5 minutes to a total maximum dose of 0.04 mg/kg (3 mg) in the adult. Atropine is also effective when administered via the endotracheal route. The suggested endotracheal dose is 2 to 2.5 times the recommended intravenous dose diluted in 10 mL of normal saline. Because atropine can increase myocardial oxygen consumption, it should be used with caution in the presence of myocardial ischemia. Adverse effects include sinus tachycardia, ventricular tachycardia, or ventricular fibrillation, as well as manifestations of anticholinergic toxicity. In patients with high-degree AV block, one may also see, in rare instances, a marked reduction in the heart rate after atropine use.
Glucagon has been found to be beneficial in treating bradydysrhythmias secondary to β-blockers and calcium channel blockers. The drug appears to be effective in both toxic and nontoxic patients. Glucagon stimulates the SA node, resulting in a modest increase in the heart rate. Glucagon increases automaticity at the AV node, which may be helpful in slow junctional rhythms. Glucagon has also been shown to increase cardiac contractility, increasing coronary perfusion. The peak action of glucagon occurs approximately 5 to 10 minutes after intravenous administration. Suggested initial dosing ranges are 0.05 to 0.10 mg/kg in children and 0.05 to 0.15 mg/kg in adults. This dose may be repeated based on the clinical situation and the characteristics of the offending medication.
Isoproterenol, a β-adrenergic sympathomimetic drug with potent inotropic and chronotropic effects, is only used rarely currently, having been largely supplanted by the widespread availability of transcutaneous pacing. Still, isoproterenol may be useful as a temporary measure when other options fail. It is administered as an infusion at a rate of 2 to 10 mg/min. Isoproterenol markedly increases myocardial oxygen demand and may cause significant adverse effects, including ventricular tachycardia or fibrillation.
If drug therapy is ineffective, artificial pacing may be instituted. Transcutaneous pacing is more easily performed than internal pacing and should be attempted first. Emergent temporary pacing may improve organ perfusion and cognitive function (12).
Disorders of Impulse Formation
Atrial Bradydysrhythmias
Sinus dysrhythmia is a physiologic finding commonly seen in healthy young people. In it, the sinus discharge rate decreases with expiration and increases with inspiration. Sinus dysrhythmia is thought to be caused by changes in vagal tone during respiration. P wave morphology and P–R intervals are usually constant. No treatment is indicated.
Sinus bradycardia, arbitrarily defined as a sinus rhythm of <60 bpm, may be the result of organic heart disease and may cause symptoms, but it is also a common finding in healthy patients and particularly in conditioned athletes (13). The significance of sinus bradycardia depends on the clinical setting. It is common during therapy with digitalis and β-blockers and may not warrant intervention. In contrast, the following findings should suggest nonphysiologic sinus bradycardia: (i) profound bradycardia in an individual not engaged in strenuous endurance training, (ii) sinus pauses longer than 3 seconds on a Holter monitor, or (iii) syncope or near-syncope at rest or after strenuous exercise. When sinus bradycardia is symptomatic, treatment with atropine is usually successful, although cardiac pacing is sometimes necessary.
The sick sinus syndrome encompasses a spectrum of conditions, including severe sinus bradycardia, SA block, sinus arrest, and the bradycardia–tachycardia syndrome. The latter refers to the intermittent occurrence of bradydysrhythmias and tachydysrhythmias (e.g., atrial fibrillation, flutter, or paroxysmal supraventricular tachycardia) in the same patient. The bradydysrhythmia typically occurs immediately after resolution of an episode of tachycardia. Symptoms such as syncope or chest pain may result from either the tachycardia or the bradycardia. Pharmacologic therapy that is effective for the tachycardia may exacerbate the bradycardia, so cardiac pacing may need to be initiated before proceeding with pharmacologic therapy.
Atrioventricular Nodal Bradydysrhythmias
An AV junctional rhythm may be a physiologic rhythm initiated by the AV node in the absence of an adequate sinus stimulus or may result from an abnormally rapid AV junctional focus that usurps control from the sinus node (e.g., accelerated junctional rhythm). Junctional rhythms usually exhibit a QRS morphology similar to the patient’s sinus rhythm. The P waves are usually inverted if they are conducted in a retrograde manner. The P waves can fall before, during, or after the QRS complex, depending on the location of the focus within the AV junction and the degree of retrograde AV block. When the P wave occurs before the QRS complex, the P–R interval is usually <0.12 seconds. The absence of P waves may be caused by obliteration by the QRS complex or by retrograde block.
AV junctional escape beats occur singly or multiply in the absence of stimuli arriving at the AV node (Fig. 84.1). Their hallmark is occurrence after an interval longer than the dominant cycle. The underlying disorder may be sinus bradycardia, sinus arrest, sinus exit block, or AV block. Digitalis or β-blocker therapy may also be responsible. Junctional escape rhythms may also be incidental findings in otherwise healthy people with increased vagal tone. Treatment is not indicated for asymptomatic patients with infrequent escape beats. If symptoms occur, the clinician should treat the underlying rhythm rather than attempt to obliterate the escape beats, as pharmacotherapy to obliterate the AV nodal escape beats may lead to asystole. Treatment involves withholding offending drugs or using atropine or artificial pacing.
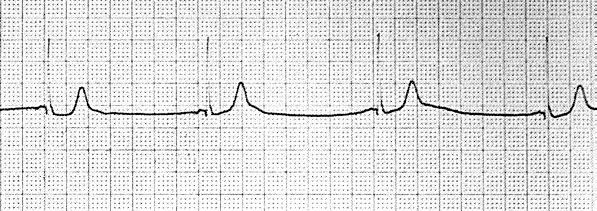
FIGURE 84.1 Junctional escape rhythm.









