BIOLOGICAL AND CHEMICAL TERRORISM*
RICHARD J. SCARFONE, MD, JAMES M. MADSEN, MD, MPH, FCAP, FACOEM COL, MC-FS, THEODORE J. CIESLAK, MD, FRED M. HENRETIG, MD, AND EDWARD M. EITZEN JR., MD, MPH
GOALS OF EMERGENCY CARE
Biologic and chemical terrorism involves the use of highly virulent or toxic agents with the intent to cause mass casualties, which could overwhelm regional emergency medical services (EMS) capacity and would pose unique medical management challenges. Treatment goals include early syndrome recognition, understanding specific pediatric vulnerabilities, and knowing the major biologic and chemical agents of concern and the management of children exposed to them.
KEY POINTS
 Even small-scale, technologically primitive biologic or chemical attacks can cause considerable morbidity and wreak havoc on regional medical care systems, thereby successfully terrorizing a population. Examples include the intentional spread of salmonella in Oregon restaurants in 1984; the 1995 sarin release on Tokyo subways; and the anthrax release in US mail in 2001.
Even small-scale, technologically primitive biologic or chemical attacks can cause considerable morbidity and wreak havoc on regional medical care systems, thereby successfully terrorizing a population. Examples include the intentional spread of salmonella in Oregon restaurants in 1984; the 1995 sarin release on Tokyo subways; and the anthrax release in US mail in 2001.
 Chemical attacks result in almost immediate effects, whereas biologic attacks evolve over days to weeks based on the incubation period of the infectious agent used.
Chemical attacks result in almost immediate effects, whereas biologic attacks evolve over days to weeks based on the incubation period of the infectious agent used.
 Children have several unique vulnerabilities, including the following:
Children have several unique vulnerabilities, including the following:
 Risk of exposure—increased respiratory and dermal exposure
Risk of exposure—increased respiratory and dermal exposure
 Physiologic response—increased risk of dehydration and hypothermia
Physiologic response—increased risk of dehydration and hypothermia
 Psychological response—less ability to cope with stress and emotional trauma
Psychological response—less ability to cope with stress and emotional trauma
 Systems vulnerabilities—EMS and ED providers may feel less comfortable taking care of children
Systems vulnerabilities—EMS and ED providers may feel less comfortable taking care of children
RELATED CHAPTERS
Signs and Symptoms
• Respiratory Distress: Chapter 66
Medical, Surgical, and Trauma Emergencies
• Environmental Emergencies, Radiological Emergencies, Bites and Stings: Chapter 98
• Infectious Disease Emergencies: Chapter 102
• Pulmonary Emergencies: Chapter 107
BIOLOGIC AGENTS
CLINICAL PEARLS AND PITFALLS
• Biologic attacks should be suspected when there are an unusually high number of cases, a common exposure history, and exotic disease presentations.
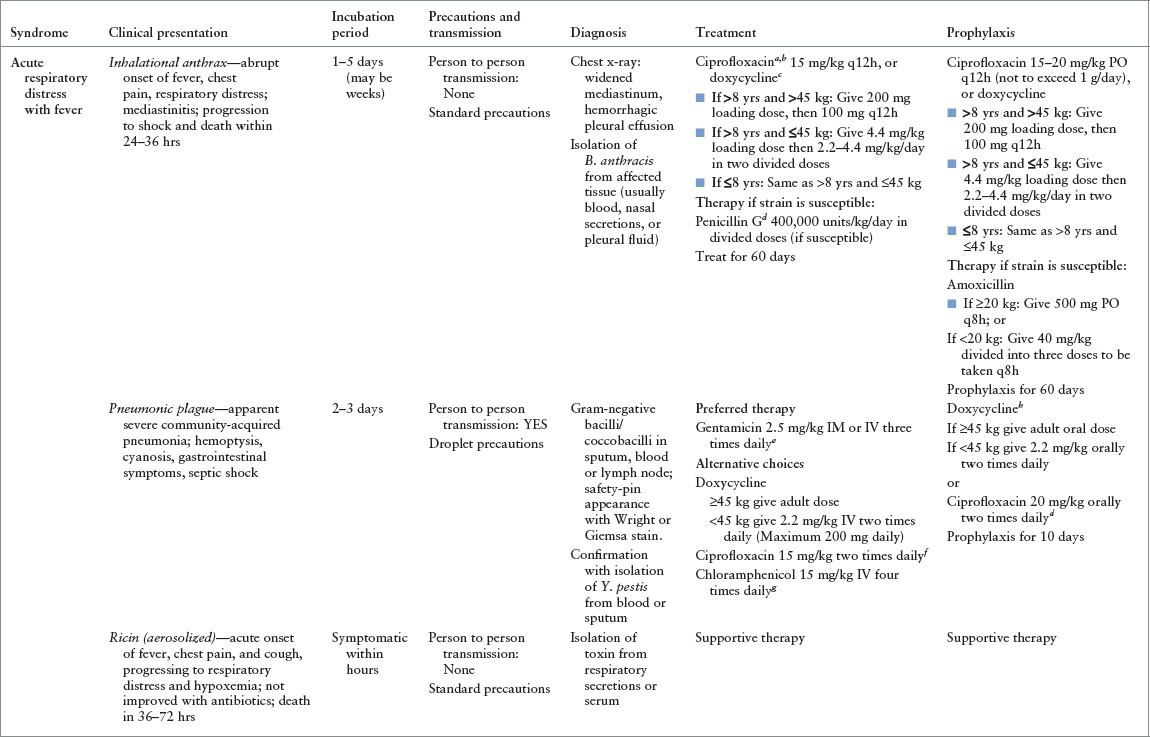
• Ciprofloxacin, levofloxacin, and doxycycline are currently considered drugs of choice in the treatment and prophylaxis of anthrax, plague, and tularemia, even in children (Tables 136.1 and 136.2).
Current Evidence
A working group convened by the Centers for Disease Control and Prevention (CDC) has identified anthrax, smallpox, plague, botulism, tularemia, and the viral hemorrhagic fevers as the biologic diseases that would constitute the gravest threats to public health and security; the causative microorganisms are termed Category A agents. We thus limit our focus here to these six agents (Table 136.3). In addition we add a brief discussion of the phytotoxin (plant toxin) ricin because of its ready availability and ease of production. Treatment protocols for these uncommon conditions are likely to evolve continuously, particularly if future incidents occur, as was the case when the mail-borne anthrax outbreak unfolded.
Of note, the fluoroquinolones and/or tetracyclines are currently considered drugs of choice in the treatment and prophylaxis of anthrax, plague, and tularemia. Although these have been little used by pediatricians in the past, there is now considerable recent experience with the use of these antibiotics for selected serious pediatric infections. Furthermore, the risk of morbidity and mortality from these biologic agent–induced diseases far outweighs the minor risks (arthropathy with fluoroquinolones, dental staining with tetracyclines) associated with short-term pediatric use of these medications. In fact, ciprofloxacin, levofloxacin, and doxycycline have lower risk of these adverse effects and are approved by the U.S. Food and Drug Administration (FDA) for use in children for the treatment and prophylaxis of anthrax and plague following inhalational exposure (i.e., in the context of terrorism).
TABLE 136.1
CHARACTERISTICS OF CHEMICAL AND BIOLOGIC ATTACKS
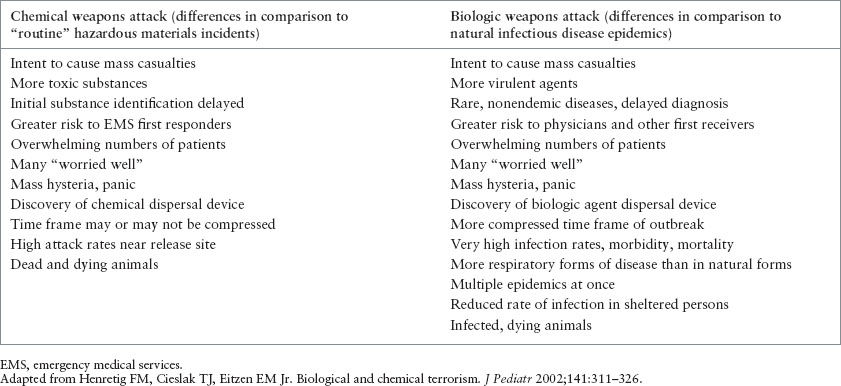
TABLE 136.2
PEDIATRIC VULNERABILITIES TO BIOLOGIC AND CHEMICAL TERRORISM
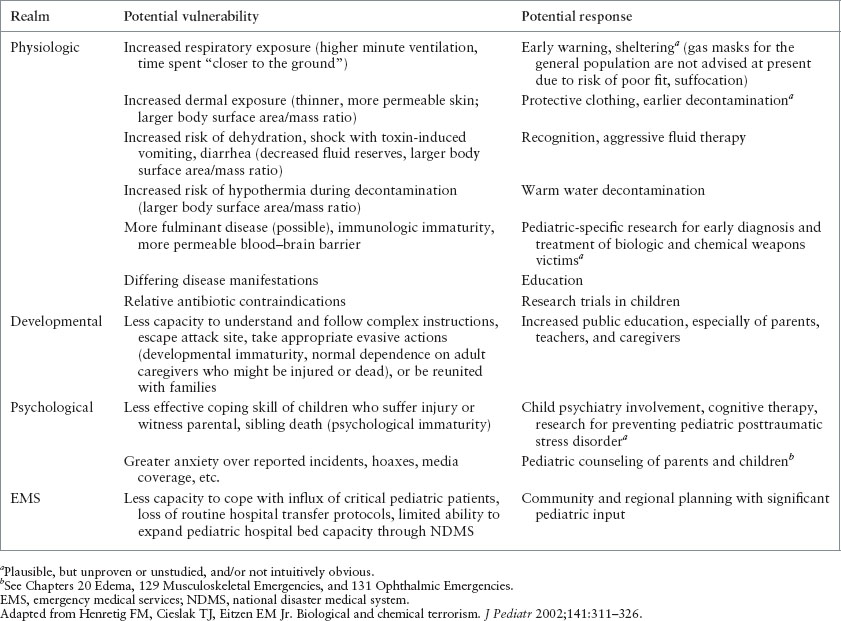
TABLE 136.3
PRIMARY THREAT BIOLOGIC AGENTS OF TERRORISM

Specialized laboratory services would likely be required to rapidly identify biologic agents or confirm a diagnosis of disease caused by them. Further, work with most of these agents is very hazardous. As such, a national Laboratory Response Network (LRN) has been established to facilitate the timely detection of bioterrorism-related diseases. This network involves laboratories at local, state, and federal levels. The latter include the CDC and the U.S. Army Medical Research Institute of Infectious Diseases (USAMRIID) at Fort Detrick in Frederick, Maryland. If pediatric emergency care providers suspect such an illness, they should immediately inform their hospital microbiology laboratory and infection control office as well as the local public health departments, to effect prompt notification and transport of specimens to the appropriate laboratory.
Goals of Treatment
The goals of emergency therapy include early recognition of a potential biologic agent attack, efficient and accurate triage of victims, proper isolation and infection control precautions to protect healthcare workers and other patients, and administration of proper antibiotics, when appropriate.
Clinical Considerations
Clinical Recognition
Pediatricians and ED physicians rarely encounter victims of biologic agent exposure. Considering three critical epidemiologic characteristics of such an attack might enhance early recognition: an epidemic number of patients, a common exposure history, and exotic disease presentations. A large number of patients, out of proportion to time of year and expected clinical syndromes, might trigger suspicion. Although some variations in the incubation period may occur after a biologic agent attack, most persons would initially be exposed at the same time, and thus become ill and present in a relatively compressed time frame. In contrast, most natural epidemics evolve with a gradual rise in disease incidence because persons are progressively exposed to increased numbers of infectious patients, fomites, or vectors that spread the organism.
A history of geographic connection among patients, or some observation of an unusual source of exposure such as a powder in an envelope, might also sound the alarm. By exotic diseases, it is suggested that many infections caused by biologic weapons, particularly with advanced disease, are relatively unusual and unique. Diseases that are rare, not endemic in the area of exposure, or that are normally spread by vectors that are not indigenous to the relevant geographic area would also be suspect, especially if numerous cases developed simultaneously.
Additional clues to a biologic agent attack might include especially high infection rates among exposed persons, high rates of patients with atypical pneumonia, particularly high morbidity or mortality, simultaneous epidemics caused by different pathogens, attack rates lower in persons sheltered from the suspected route of exposure, presence of infected or dying animals, and the discovery of suspicious actions or potential delivery systems.
Most of the primary biologic threat agents can be categorized as causing the subacute onset of effects (e.g., days after exposure) and further divided into predominantly respiratory, neurologic, or dermatologic syndromes. Thus, with a careful medical and epidemiologic history, physical examination, and limited, routine laboratory evaluation, an early suspicion of a biologic attack might be raised, and initial diagnostic impression considered, as outlined in Figure 136.1. This in turn could trigger appropriate requests for infectious disease consultation and more definitive laboratory testing, as well as early empiric therapy. A similar approach, applied universally with unusual increases in patient volume or illness presentations, might also help practitioners to participate in the early recognition of a new or reemerging natural infectious disease (e.g., West Nile disease, severe acute respiratory syndrome [SARS], Middle East Respiratory Syndrome [MERS], monkeypox, and pneumonic tularemia, to name more recent examples). If a pediatrician recognizes, or even suspects, any such natural or intentional outbreak, immediate reporting to local and regional public health authorities is appropriate, even before a specific diagnosis can be confirmed.
Triage Considerations: Minimizing Spread of Infection
As soon as ED staff suspect that a patient may be the victim of biologic terrorism, appropriate steps must take place to prevent or minimize exposure to limit the spread of disease. The level of ED mitigation and preparedness activities will largely depend on the level of awareness of the disease outbreak. For example, if society were faced with a known release of smallpox by terrorists, EDs would need to take dramatic steps to protect staff and patients. Such steps might include setting up screening stations outside of the hospital, staffed by clinicians wearing gowns, gloves, N-95 respirators, and eye protection. If a child suspected to have smallpox were encountered at the screening station, he or she would need to be covered with a sheet, provided a mask, and escorted directly to a negative-pressure room for further evaluation and treatment. Infection Prevention and Control specialists would need to provide guidance on specimen collection, handling, and testing. Patients suspected to have smallpox would need to be cohorted into specific units of the hospital or in dedicated facilities.
At the opposite end of the spectrum would be the inadvertent discovery of a patient suspected to have an infectious disease. Perhaps an ED triage nurse discovers that within a short period of time two separate children arrive with complaints of floppiness and weakness, raising the concern for botulism. Perhaps a pediatric resident becomes concerned about a viral hemorrhagic fever after encountering a highly febrile and ill-appearing child with a purpuric rash who recently traveled to Africa. Although not all of the Category A biologic agents are spread from person to person (Table 136.3), in these cases it would be prudent to assume this mode of spread. The staff member, after washing his hands, should put on a gown, gloves, an N-95 respirator, and eye protection. The child should be covered with a sheet, provided a mask, and escorted directly to a negative-pressure room for further evaluation and treatment.
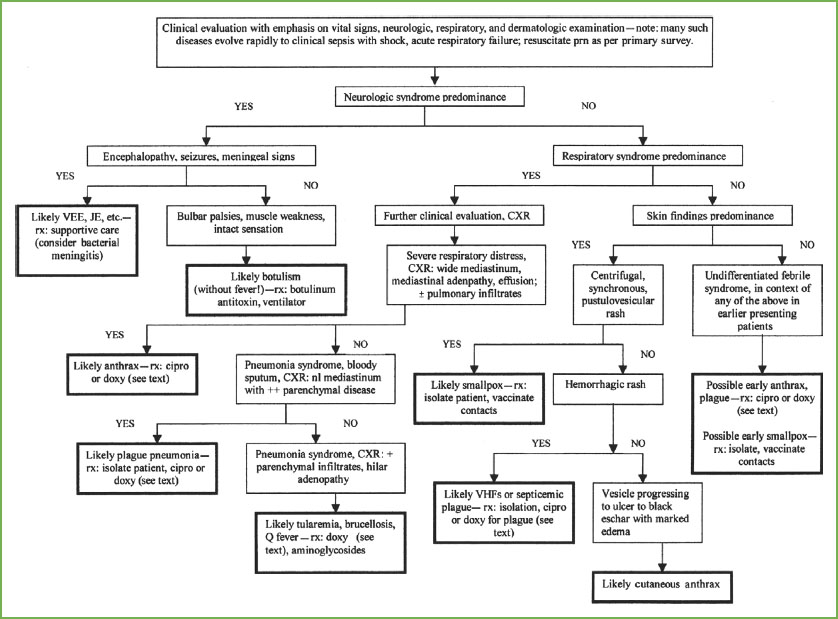
FIGURE 136.1 Approach to the early recognition and diagnosis of an attack with an unknown biologic agent. VEE, Venezuelan equine encephalitis; JE, Japanese encephalitis; Rx, treatment; CXR, chest x-ray; VHF, viral hemorrhagic fever. (Reprinted from Henretig FM, Cieslak TJ, Kortepeter MG, et al. Medical management of the suspected victim of bioterrorism: an algorithmic approach to the undifferentiated patient. Emerg Med Clin N Amer 2002;20:351–364, with permission from Elsevier.)
Clinical Assessment and Management
Specific Agents
Anthrax
CLINICAL PEARLS AND PITFALLS
• Inhalational anthrax should be suspected when there is fever with a widened mediastinum on chest x-ray.
• Anthrax is not contagious and therefore poses no risk to healthcare workers.
Background. Anthrax is caused by infection with Bacillus anthracis, a gram-positive spore-forming rod capable of surviving long periods in its spore form without nutrients or moisture. Natural disease caused by B. anthracis manifests in cutaneous, gastrointestinal (GI), and inhalational forms. Anthrax spores can be formulated in a manner to enhance aerosolization. The resulting small particles may drift long distances with air currents, produce lethal infection when inhaled, and resist environmental degradation, making them a formidable terrorist weapon.
The anthrax attack of 2001 was characterized by 22 confirmed or suspect cases (11 inhalational, 11 cutaneous), with five deaths, resulting from presumed or known exposure to anthrax-contaminated mail. The attack resulted in enormous public anxiety, as well as major demands for medical care and public health resources. Antibiotic prophylaxis was prescribed for more than 30,000 persons, and decontamination of the Hart Senate Office Building alone took months and cost an estimated $23 million. Many bioterrorism defense experts, however, fear an even more widespread aerosol release that could potentially sicken hundreds of thousands.
Inhalational anthrax is the disease form that poses the greatest threat. Following the accidental release of anthrax spores from a Soviet military facility at Sverdlovsk in 1979, 66 of 77 known victims of inhalational anthrax died. In the recent U.S. attack, all 5 deaths were among the 11 patients with this form of disease.
Pathophysiology/common manifestations. Inhalational anthrax results from spore uptake in the alveoli by pulmonary macrophages, followed by bacterial germination and toxin production in the mediastinal lymph nodes, leading to hemorrhagic lymphadenitis, mediastinitis, and sepsis. Symptoms typically begin 1 to 5 days after exposure, although incubation periods up to several weeks in length have been reported. The disease begins as a nonspecific influenza-like illness, characterized by fever, headache, myalgia, and cough. The relative lack of eye, nose, and throat findings such as red, watery eyes, rhinorrhea, or pharyngitis helps to distinguish this phase from common viral infections. A brief intervening period of improvement sometimes follows, but rapid deterioration then ensues with high fever, dyspnea, cyanosis, and shock marking this second phase. Hemorrhagic meningitis occurs in up to 50% of cases. Chest radiographs or computed tomography scans may reveal a widened mediastinum or prominent mediastinal lymphadenopathy; infiltrates and pleural effusions may also be seen. Gram stains of peripheral blood smears may demonstrate the bacterium at this stage. Prompt treatment is imperative because historically death occurred in as many as 95% of inhalational anthrax cases if such treatment began more than 48 hours after symptom onset. Even with modern intensive care, in the 2001 anthrax attack, all four patients with inhalational anthrax who exhibited signs of fulminant disease prior to antibiotic administration died. Thus, in the context of a known bioterrorism incident, a potential dilemma facing emergency care providers involves deciding which patients presenting with nonspecific flu-like, febrile illness are candidates for empiric antibiotic therapy.
Cutaneous anthrax occurs when organisms gain entry into skin, usually through abrasions or cuts. It is characterized by the appearance of a papule at the inoculum site, which then progresses over days to a vesicle, then to an ulcer, and finally to a depressed, black eschar. The surrounding tissue becomes markedly edematous, but not particularly tender, distinguishing this infection from typical cellulitis. This form of anthrax is quite amenable to therapy with a variety of antibiotics and, with timely institution of treatment, is rarely fatal. In the 2001 outbreak, all 11 patients with cutaneous anthrax survived. The one pediatric victim of the 2001 attack was a 7-month-old boy with cutaneous anthrax on his arm, presumably contracted after a brief visit to a New York City television news studio that had received contaminated mail (a similar lesion is pictured on the face of a child in Fig. 136.2). Of note, he also developed hemolysis, thrombocytopenia, and renal insufficiency, features not usually observed in otherwise uncomplicated cases of cutaneous disease, thus raising the possibility of a particular vulnerability in infancy.
The finding of gram-positive rods in skin biopsy material (in the case of cutaneous disease) or in blood smears, pleural fluid, or spinal fluid should suggest anthrax. Chest radiographs demonstrating a widened mediastinum in the context of fever and constitutional signs should also lead one to consider the diagnosis. Confirmation may be obtained by blood culture. State health laboratories, USAMRIID, and the CDC can also confirm a diagnosis of anthrax by polymerase chain reaction (PCR) and immunohistochemical assay.
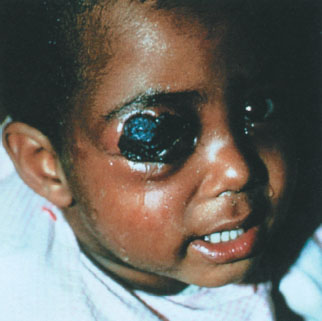
FIGURE 136.2 Cutaneous anthrax on the eyelids of a young child. (Courtesy of Dr. Larry Schwab. Reprinted from Ostler HB, Maibach HI, Hoke AW, et al. Diseases of the eye and skin: a color atlas. Philadelphia, PA: Lippincott Williams Wilkins, 2004, with permission.) (see the color-tip insert.)
Management. As anthrax has little potential for person to person transmission, standard precautions are adequate for healthcare workers caring for anthrax victims. Given the usual 1- to 5-day incubation period, decontamination of victims presenting days after exposure is unwarranted. Aerosolization of organisms from skin or clothing likewise poses little threat under typical circumstances, but bathing and laundry with soap and water would seem prudent soon after direct physical contact with a suspect substance.
Although naturally occurring strains of B. anthracis are usually quite sensitive to penicillin G, penicillin-resistant strains of B. anthracis are known; thus, many experts consider ciprofloxacin (10 to 15 mg per kg, max 400 mg, IV q12h), levofloxacin (8 mg per kg, max 500 mg, IV q12h), or doxycycline (2.2 mg per kg, max 100 mg, IV q12h) as essential components of first-line treatment for victims of intentional anthrax. Infectious Disease and Emergency Preparedness experts can provide advice regarding postexposure prophylaxis. See Table 136.3 for detailed treatment recommendations for children. Newer treatments include the recently licensed monoclonal antibody raxibacumab, and investigational anthrax immune globulin preparations.
Plague
Background. Plague, caused by infection with the gram-negative bipolar-staining rod Yersinia pestis, is usually transmitted in nature via the bite of fleas. Endemic disease is still seen in areas of the southwestern United States, South and Southeast Asia, as well as in South America and Africa. Plague has long appeared attractive as an agent of bioterrorism. Testimony to its extreme lethality and infectivity can be obtained by considering that the “Black Death” eliminated one-third of the population of Europe during the Middle Ages.
Pathophysiology. Y. pestis is a facultative intracellular pathogen that is able to survive temporarily within macrophages, thus aiding its dissemination to distant sites following inoculation or inhalation. It is lymphotropic, and significantly tender regional lymphadenopathy (e.g., in the distribution of a flea bite) is often a prominent feature of bubonic plague. Pneumonic plague (along with smallpox) is one of the few bioterrorist threats readily transmissible from person to person via the respiratory route, and coughing patients are often highly contagious. However, because plague, unlike smallpox, is spread by large respiratory droplets, close contact is needed for transmission.
Clinical manifestations. Bubonic plague is characterized by the classic bubo, a tender, enlarged, fluctuant lymph node in the distribution of the infected flea bite. Fever and malaise are usually present. Bubonic plague may progress to septicemia as bacteria gain access to the circulation; 80% of bubonic plague victims have positive blood cultures. Petechiae, purpura, and overwhelming disseminated intravascular coagulation (DIC) may develop.
Pneumonic plague may arise secondarily after blood-borne seeding of the lungs or may be seen primarily after aerosol exposure. Symptoms include high fever, chills, malaise, fatigue, headache, and cough. Chest radiographs may reveal a patchy or consolidated bronchopneumonia, and the classic clinical finding is one of blood-streaked sputum; DIC and an overwhelming sepsis may develop as the disease progresses. Meningitis develops in 6% of cases. Untreated pneumonic plague has a mortality rate approaching 100%.
A presumptive diagnosis of plague can be made by observing the classic bipolar-staining “safety pin”–like rods in Gram or Wayson stains of sputum, aspirated lymph node material, or cerebrospinal fluid. Confirmation is obtained via blood, sputum, or aspirate culture.
Management. Droplet precautions should be employed in cases of suspected pneumonic plague. Such precautions should be continued in confirmed cases until sputum cultures are negative. Standard precautions are adequate in managing bubonic plague victims. Given the incubation period, decontamination would not be necessary in a clinical setting. See Table 136.3 for detailed treatment recommendations for children.
Smallpox
CLINICAL PEARLS AND PITFALLS
• Smallpox is contagious 1 day prior to the onset of rash.
• In contrast to chickenpox, the rash of smallpox starts on the face and extremities and spreads centrally.
• The smallpox rash develops synchronously such that lesions are all in the same stage.
• Strict airborne, droplet, and contact precautions should be instituted immediately for smallpox victims and should continue until all scabs have separated.
• Smallpox is associated with a high mortality and there is no specific therapy available.
Background. The global eradication of smallpox represents one of the great success stories of public health, with the last endemic case occurring in Somalia in 1977. Since then, research stockpiles of variola virus have been consolidated into two World Health Organization (WHO)–approved stores at the CDC in Atlanta and at a Russian institute in Koltsovo. This achievement would seem to make terrorist use of this virus impossible; however, several factors give cause for concern. First is the fear that other stockpiles already exist in the hands of belligerent nations unbeknownst to WHO. Astonishingly, in 2014, vials containing the smallpox virus were discovered in a storage room of an FDA laboratory in Bethesda, Maryland. Second, the entire viral genomic sequence is known and published; therefore, it is likely only a matter of time before technology permits reconstruction of the virus. Finally, although the virulence factors of variola virus are poorly understood, it may be possible for someone to manipulate related orthopoxviruses such as monkeypox to enhance their virulence in humans and create a disease similar to smallpox. In light of these considerations, the CDC in 2003 recommended a strategy of reintroducing vaccination in the United States after a nearly 30-year hiatus, with the initial goal of vaccinating up to 10,000,000 front-line EMS and healthcare providers. This program has subsequently proven controversial and has been suspended; probably fewer than 50,000 civilians were vaccinated. The U.S. military, however, has vaccinated several hundred thousand service members in recent years very successfully and serious adverse events have been rare.
Several factors might make smallpox an attractive weapon to potential belligerents. First, the duration of immunity after vaccination is a matter of controversy, with some studies suggesting a duration of only 3 to 5 years, while other studies suggest the possibility of lifelong immunity. Second, following cessation of universal vaccination within the United States in 1972, vaccine had been out of production until the 2007 licensing of a new product (ACAM2000, Acambis Corporation); although the CDC now controls enough ACAM2000 to vaccinate every American, susceptibility to the disease is nearly universal. Also, effective therapy is lacking and healthcare providers are unfamiliar with the disease. Finally, the potential for rapid spread potentially permits a terrorist to cause widespread disease and panic with a minimum of infectious material.
Pathophysiology. Although infectivity is highest when the smallpox rash first appears, the disease may be spread by exposed persons about 24 hours before the exanthem manifests. During the 7- to 17-day long incubation period, the virus replicates in upper respiratory tract mucosa, giving rise to a primary viremia. The liver and spleen are then seeded, further amplification of the virus occurs, and a secondary viremia ultimately develops. The skin is seeded with this secondary viremia, and the classic exanthem of smallpox develops.
Clinical manifestations. Clinical illness has an abrupt onset during the phase of secondary viremia and is characterized by fever, malaise, rigors, vomiting, headache, and backache. The classic exanthem typically begins 2 to 4 days later as macules on the face and extremities (Fig. 136.3). These lesions progress in synchronous fashion to papules, then to pustules, and finally form scabs. As scabs separate, survivors are left with disfiguring depigmented scars. The rash spreads centrally to the trunk but remains more abundant at the periphery. This centrifugal distribution and synchrony distinguish smallpox from chickenpox, which has a centripetal distribution of lesions in varying stages of development. An enanthem usually accompanies the characteristic exanthem, and internal organs become viral targets as well. Death occurs in 30% of variola major (the predominant form of smallpox in the past) patients and typically results from hypotension and immune complex–associated toxemia. Eye involvement leads to blindness in a small number of victims. Uncommon variants with lesser (variola minor) or greater (hemorrhagic and flat-type variants) mortality also existed. Ominously, a 1971 smallpox outbreak in Aralsk, in the former Kazakh Soviet Republic, is thought by some to have originated from a bioweapons accident. All three fatalities in this small outbreak involved unvaccinated individuals who contracted rare and universally fatal hemorrhagic disease, raising the specter of genomic manipulation leading to increased virulence.
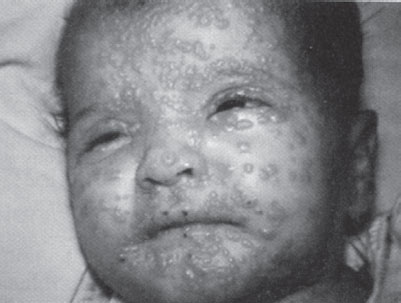
FIGURE 136.3 A child with smallpox. (Reprinted from Henretig FM, McKee MR. Preparedness for acts of nuclear, biological and chemical terrorism. In: Gausche-Hill M, Fuchs S, Yamamoto L, eds. APLS: the pediatric emergency medicine resource. American Academy of Pediatrics and American College of Emergency Physicians. Sudbury, MA: Jones and Bartlett, 2004:568–591, with permission.)
Management. The diagnosis of smallpox should be suspected on clinical grounds. Laboratory confirmation (e.g., electron microscopy, PCR) is best effected in a biosafety level 4 laboratory by emergent notification and specimen transport to the CDC.
Based on past experience, vaccination (with vaccinia, an orthopoxvirus closely related to variola) of smallpox-exposed persons within the first 4 days after exposure may prevent the development of overt disease. Although the vaccine has been used safely and successfully in even young infants, it has a relatively high rate of serious complications in certain patients. Notably, fetal vaccinia and resultant fetal demise can occur when pregnant women are vaccinated. Vaccinia gangrenosa, a frequently fatal complication, occurred when immunocompromised persons were inadvertently vaccinated. Eczema vaccinatum may occur in those with pre-existing skin conditions and can be serious. Myocarditis and pericarditis may occur. A severe postvaccinal encephalitis was well known, albeit relatively rare, during the era of widespread vaccination, because this complication occurs only after primary vaccination, it would disproportionately affect pediatric patients. Autoinoculation can occur when virus from the primary lesion arising at the site of vaccination is transferred by scratching or rubbing to other areas of the skin or to the eye. To manage these complications, vaccinia immune globulin (VIG) should be available when undertaking a vaccination campaign. VIG (0.6 mg per kg intramuscular [IM]) may be given to vaccine recipients who experience severe complications or to significantly immunocompromised individuals exposed to smallpox in whom vaccination would be unsafe. Today, stocks of vaccine and VIG are controlled by the CDC.
Even a single case of smallpox occurring anywhere in the world today would represent a grave public health emergency. A suspected case should thus prompt immediate notification and consultation with health authorities. Strict airborne, droplet, and contact precautions should be instituted immediately for victims and should continue until all scabs have separated. Decontamination of symptomatic patients is unnecessary. Contacts must be observed closely for 17 days following their last potential exposure. The development of fever during this period would be cause for isolation. Multiple victims would ideally be managed as a cohort at dedicated sites removed from conventional hospital facilities.
Botulism
Background. Botulism occurs as a result of exposure to one of eight botulinum neurotoxins (A through H). Only types A, B, E, and, rarely, F appear to cause human botulism in nature. Botulinum toxin was included in the U.S. biologic arsenal in the 1950s and 1960s, and was weaponized by Iraq in the 1980s. The Aum Shinrikyo cult in Japan tried unsuccessfully to disseminate botulinum toxin before deciding to release sarin in the Tokyo subway system.
Pathophysiology. Botulinum toxins are produced by certain strains of Clostridium botulinum, a strictly anaerobic spore-forming gram-positive rod commonly found in soil. Most cases of natural botulism result from ingestion of preformed toxin (food poisoning) or intestinal toxin formation (infant form). Infant botulism has additional unique epidemiologic considerations; more extensive discussion of this form, and of botulism in general, may be found elsewhere in this text (see Chapters 78 Weakness and 105 Neurologic Emergencies). The botulinum neurotoxins are the most toxic substances known to man. These toxins function at the peripheral cholinergic presynaptic nerve terminals, principally the neuromuscular junction, by preventing the release of acetylcholine and thereby leading to a generalized flaccid paralysis and autonomic symptoms. In keeping with the fact that toxins are chemical poisons produced by biologic organisms, it is important to keep in mind that cases of botulism arising from a terrorist attack represent chemical intoxication rather than an infectious disease caused by replicating C. botulinum organisms.
Clinical manifestations.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







