Fig. 14.1
Plain abdominal radiograph of a patient with gallstone ileus. Note the presence of pneumobilia depicted in the circled area. Gallstones may not be present due to lack of calcium composition
Ultrasound
Ultrasound has become the initial diagnostic test of choice in patients with suspected biliary disease. The test can be rapidly performed at the patient’s bedside and does not require the use of radiation. Ultrasound is highly accurate for identifying stones that are ≥5 mm in size (>96 %) [32]. In order to detect stones, they must be echogenic, have posterior acoustic shadowing, and be mobile (see Fig. 14.2). False-negative results may be seen with decreased sonographer experience, large amounts of bowel gas, small stone size (<3 mm), or with soft pigmented stones (“brown stones”) [32–34]. Examining the gallbladder with the patient in multiple different scanning positions can lower the rate of false negative exams.
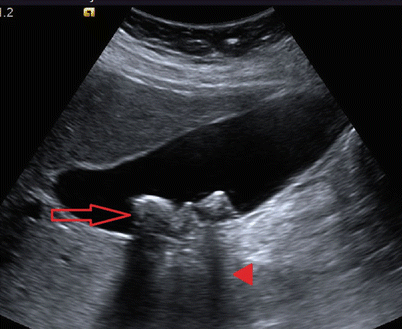

Fig. 14.2
Abdominal ultrasound documenting cholelithiasis. Note the hyperechoic stones (arrow) and posterior acoustic shadowing (arrowhead)
Ultrasonography is also helpful in establishing the diagnosis of acute and chronic cholecystitis. In the setting of acute cholecystitis, the most reliable finding is a sonographic Murphy’s sign or tenderness over the gallbladder with transducer pressure. This finding is 87 % specific for the diagnosis of acute cholecystitis and has a positive predictive value of 92 % when stones are also visualized [35]. False-negative sonographic Murphy’s sign may occur in patients that are immunosuppressed, obtunded, recently medicated, or have denervated gallbladders (i.e., diabetics or gangrene of the gallbladder) [32]. Other findings that are indicative of acute cholecystitis include gallbladder wall thickening (>3 mm), which is present in 50 % of cases, as well as the presence of pericholecystic fluid (see Fig. 14.3) [32, 35]. It should be noted, however, that these findings are nonspecific and may occur with adjacent right upper quadrant pathology. The ultrasonographic diagnosis of chronic cholecystitis can also be suggested by nonspecific gallbladder wall thickening due to fibrosis with resultant contraction and near obliteration of the gallbladder lumen producing the “double arc” sign [36].
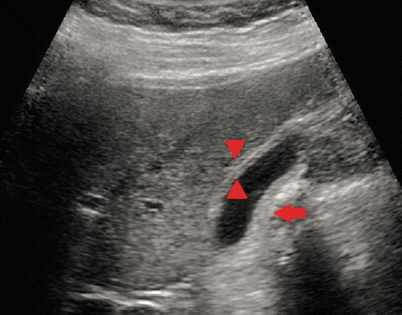

Fig. 14.3
Ultrasound depicting the findings of acute cholecystitis. Note the presence of gallbladder wall thickening (double arrowhead) and pericholecystic fluid (single arrow)
It should also be noted that right upper quadrant ultrasound is also the initial imaging study of choice to screen for choledocholithiasis. It allows for quick assessment of the bile duct size and continuity. The extrahepatic common bile duct should be measured at the level of the right hepatic artery and not exceed 6 mm, while the intrahepatic bile ducts should not exceed 2 mm in size [34]. With adequate sonographer experience, the level of biliary obstruction can be identified in 92 % of patients, and overall sensitivity for choledocholithiasis can reach 75 % [34]. It is important to emphasize that choledocholithiasis can also be present in the absence of biliary ductal dilation in 25–33 % of cases [37]. When this occurs or when stones are less than 5 mm in diameter combined with overshadowing by bowel gas, the sensitivity of ultrasound drops considerably. Endoscopic ultrasonography has considerably better sensitivity at detecting choledocholithiasis (96 %) and should be considered in select cases of presumptive biliary obstruction (i.e., low or intermediate probability of retained common duct stones) [38]. Endoscopic ultrasound has been shown to have equivalent sensitivity and specificity to endoscopic retrograde cholangiopancreatography (ERCP) and avoids not only radiation exposure but also potential complications (i.e., bleeding, perforation, and pancreatitis). In a large review of patients undergoing both endoscopic ultrasound and ERCP, Petrov and Savides [39] found that 67 % of patients could be spared ERCP with a negative ultrasound examination without any documented recurrence of common bile duct stones. Additionally, the safety of endoscopic ultrasound in elderly patients with comorbidities was demonstrated in a group of 1000 patients, which revealed that there were no age-related differences in procedure-related complications [40].
Biliary Scintigraphy (HIDA)
Biliary scintigraphy involves the administration of radiolabeled technetium iminodiacetic acid, which is taken up by the hepatic parenchyma and excreted into the bile with eventual flow into the gallbladder. The use of HIDA has largely declined into a second-line test for calculous biliary disease due to its increased expense, amount of time needed to complete the study, and the use of ionizing radiation. HIDA is considered positive for acute cholecystitis when there is the absence of gallbladder visualization within 60 min (see Fig. 14.4). This test can be carried out in a delayed fashion for up to 4 h; nonvisualization during this extended time frame is considered consistent with chronic cholecystitis [41]. Scintigraphy has excellent diagnostic sensitivity (>95 %), particularly in nonhospitalized patients that are much less likely to have false-positive imaging studies [41]. False-positive studies may be seen in up to 30–40 % of patients that are hospitalized for a reason other than abdominal pain, which is a common scenario in the elderly population [42]. Reasons for false-positive exams include: prolonged fasting, cholestasis secondary to hepatic disease, or prolonged parenteral nutrition [32]. HIDA can also play a role in diagnosing postoperative biliary complications (i.e., diagnosing postoperative bile leak) as well as in those patients with suspected biliary motility disorders such as biliary dyskinesia. However, due to the previously mentioned limitations, differences in cost and radiation exposure ultrasonography should be considered as the initial diagnostic imaging test of choice for most biliary diseases.
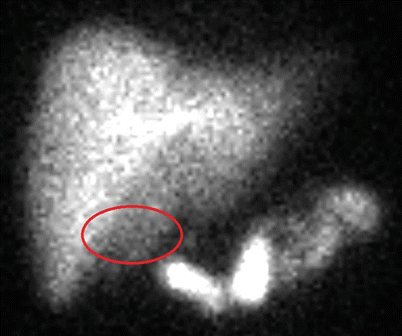

Fig. 14.4
Example of a positive HIDA scan. Note the absence of radioactivity in the area of the gallbladder fossa (highlighted oval)
Computerized Tomography
Computerized tomography (CT) has variable sensitivity in detecting gallstones secondary to the variable amount of calcification present and thus is also a second-line imaging study in the work-up of biliary disease. Those stones that are predominantly composed of cholesterol (>60 %) will be more difficult to identify due to their similar radiographic density as the surrounding bile. CT has lower sensitivity as compared to ultrasonography in identifying choledocholithiasis (75–80 %) (see Fig. 14.5) [32, 43] but can provide information regarding ductal anatomy. CT imaging is most useful in demonstrating gallbladder size, wall thickness, and surrounding inflammatory changes associated with acute cholecystitis making it highly specific (99 %) for this particular diagnosis (see Fig. 14.6) [44]. In the setting of suspected malignancy, CT is the diagnostic image of choice because it allows assessment of not only the gallbladder but also surrounding structures such as the liver, porta hepatis, identification of lymphadenopathy, or pancreaticoduodenal pathology [45]. In the elderly patient with pre-existing renal disease, diabetes, or certain medications (i.e., ACE inhibitors, NSAIDs, or metformin), caution should be taken with the administration of intravenous contrast as this can precipitate or worsen renal failure. Gentle intravenous fluid administration, sodium bicarbonate, and/or Mucomyst prophylaxis should be considered in these patients as well as ensuring that iso-osmolar contrast is administered [46]. Because of the increased cost and associated radiation exposure, CT scanning should only be considered when there is diagnostic uncertainty and other abdominal pathology is suspected [47].
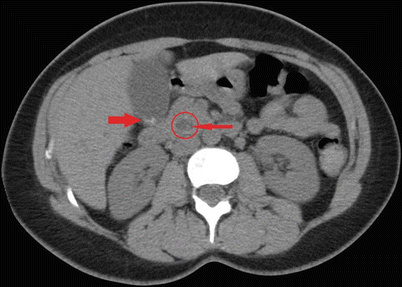
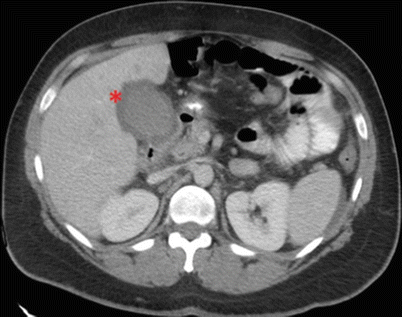

Fig. 14.5
CAT scan of the abdomen showing a stone (thin arrow) in a dilated common bile duct (circled) along with cholelithiasis (thick arrow)

Fig. 14.6
CAT scan of the abdomen showing evidence of acute cholecystitis. Notice distention of the gallbladder (asterisk) with surrounding pericholecystic fluid
Magnetic Resonance Imaging and Cholangiopancreatography (MRI/MRCP)
MRI, though not frequently used as an initial imaging test, has excellent ability to identify gallstones due to the sharp contrast in signal intensity between bile and stones on T2-weighted images [48]. This excellent resolution of stones which are as small as 2 mm in size has made MRCP the diagnostic test of choice for identifying choledocholithiasis (see Fig. 14.7) in asymptomatic patients with moderate to high probability based upon clinical examination and laboratory studies. MRCP has excellent sensitivity (81–100 %) and specificity (85–99 %) for choledocholithiasis and is comparable to ERCP in diagnostic accuracy without the invasive risk [49]. MRCP becomes less sensitive in studies with microlithiasis, pneumobilia, motion artifact, or stones in the peri-ampullary region [50]. MRI can also be useful in those with malignant disease as it images the gallbladder wall, liver parenchyma, and biliary tree with high resolution. MRI may be difficult to obtain in elderly patients with dementia or claustrophobia due to the tight confines of the imaging magnet. Also those with pacemaker or defibrillator devices may also not be candidates for MRI, though certain devices have been prospectively observed after imaging without any adverse effects [51].
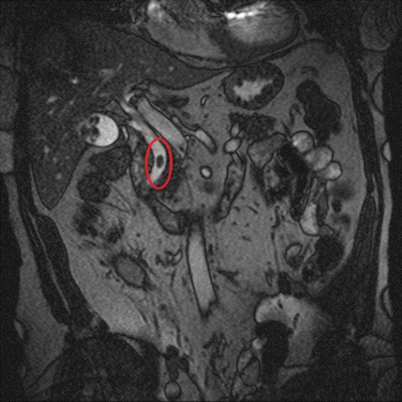

Fig. 14.7
T2-weighted MRCP showing a large stone in the common bile duct (highlighted oval)
Invasive Imaging
Endoscopic Retrograde Cholangiopancreatography
Advancements in endoscopy techniques and increased experience have made ERCP widely available, and it remains the gold standard for the diagnosis of the majority of biliary pathology. However, due to its invasive nature and improvements in the aforementioned noninvasive imaging techniques, ERCP has largely become a planned therapeutic procedure. Those patients presenting with symptomatic choledocholithiasis (i.e., pain, jaundice, fever) or with documented common duct stones on imaging are clearly potential candidates for ERCP (see Fig. 14.8). Predicting which asymptomatic patients will ultimately require ERCP is more difficult, but advanced age (>55 years), hyperbilirubinemia (>1.8 mg/dl), and common duct dilation have all been shown to increase the likelihood of a therapeutic ERCP [52]. As previously mentioned, endoscopic ultrasound prior to ERCP may eliminate a number of nontherapeutic studies. The success rate for ERCP for common bile duct procedures is near 98 % in experienced hands [53]. ERCP is associated with a number of well-described complications, with the most common being post ERCP pancreatitis, with a reported incidence ranging from 5 to 10 %. Despite the possibility of post ERCP complications, elderly patients appear to tolerate the procedure as well as their younger counterparts [54].
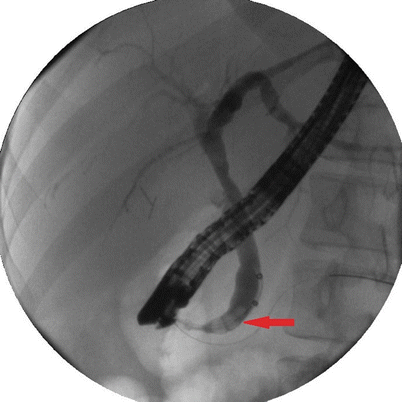

Fig. 14.8
An ERCP demonstrating a dilated common bile duct with multiple stones (arrow)
Percutaneous Transhepatic Cholangiography (PTC)
PTC involves the percutaneous passage of a needle into the liver parenchyma under fluoroscopic or ultrasound guidance and then either into the gallbladder or biliary tree for diagnostic and/or therapeutic purposes (Fig. 14.9). This technique was initially introduced in 1979 and remains a valuable option for treating biliary pathology when ERCP is either unavailable or unsuccessful, particularly in the critically ill population [55, 56]. This less invasive technique can be used to successfully treat cholangitis or surgery-related biliary complications in elderly critically ill patients with a high success rate (>95 % [57]). Alternatively, the gallbladder can be cannulated to allow decompression for high-risk patients presenting with acute cholecystitis which is known as a percutaneous cholecystostomy tube. It should be noted that PTC carries a greater complication risk than ERCP because the catheter is passed through the liver into the biliary tree. This procedure can result in post-procedure hemorrhage, septic shock from bacterial translocation, or bile peritonitis.
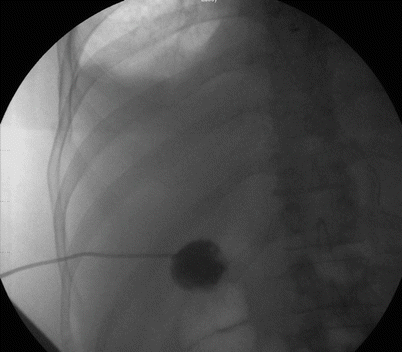

Fig. 14.9
PTC done under fluoroscopy for acute cholecystitis
Benign Calculous Diseases
Acute Calculous Cholecystitis
Clinical Presentation and Diagnosis
In the overwhelming majority of patients with acute cholecystitis, there is cystic duct obstruction by an impacted acalculous. This classically causes severe, persistent epigastric and right upper quadrant pain (especially with a positive Murphy’s sign) that may radiate to the patient’s back and be associated with nausea or vomiting. In most cases, the patient will recall more “minor” previous episodes that are in fact biliary colic, but acute cholecystitis can be the initial presentation of symptomatic gallstone disease in 15–20 % of patients [58]. In addition to the abdominal findings, there are also typically signs of systemic inflammation such as fever, tachycardia, and leukocytosis. In addition to leukocytosis, other laboratory tests that may be abnormal include: elevation of C-reactive protein and mild elevation of serum bilirubin and transaminases (<500 IU/L). Worse outcomes have been demonstrated in elderly patients presenting with LFT elevation in the setting of acute cholecystitis [59]. If the serum bilirubin is greater than 2 mg/dL, particularly the conjugated form, or serum transaminases are > 500 IU/L, choledocholithiasis should be suspected as the incidence of coexisting common bile duct stones in the elderly is high (10–20 %) [53]. It is important to emphasize, however, that the “classic” presentation in the elderly patient is often the exception rather than the rule as a significant percentage will have no fever, abdominal pain, nausea/vomiting, or normal laboratory investigation [5, 60, 61]. In the debilitated noncommunicative patient, the only presenting symptoms of acute cholecystitis will be a change in mental status or poor oral intake [61, 62].
When the diagnosis of acute cholecystitis is suspected, abdominal ultrasonography is the initial imaging test of choice. A sonographic Murphy’s sign combined with the presence of stones, gallbladder wall thickening, and pericholecystic fluid essential clinch the diagnosis. In cases where the diagnosis is less clear, or when there are no stones visualized, a HIDA scan may be useful. The CT findings of acute cholecystitis or potential gallbladder malignancy could also be assessed in patients that have this imaging test ordered for abdominal pain of unknown etiology.
Treatment of Acute Calculous Cholecystitis
Ongoing cystic duct obstruction causes inflammation and can lead to bacterial infection in the bile as well as ischemia of the gallbladder wall. Initial supportive measures such as bowel rest, intravenous fluid hydration, and analgesics are appropriate. The Infectious Diseases Society of America guidelines recommend empiric antimicrobial therapy in cases of clinically suspected infection [63]. Initial therapy should include coverage against microorganisms in the Enterobacteriaceae family. Appropriate initial antibiotic choices for noncomplicated cases include second- or third-generation cephalosporins or a combination of fluoroquinolones combined with metronidazole [63]. For patients presenting with severe sepsis or those that are considered high risk (i.e., elderly, diabetics, or the immunocompromised), broad-spectrum antibiotics such as piperacillin/tazobactam or aminoglycosides should be used [63].
The timing and choice of surgical intervention for acute cholecystitis has undergone considerable debate and change over the past several decades. Laparoscopic cholecystectomy has become the initial operative intervention of choice due to its superior outcomes compared with open surgery [64, 65]. The techniques of laparoscopic cholecystectomy are beyond the scope of this chapter and can be found elsewhere [66]. The traditional treatment approach involved initial nonoperative management with supportive measures and antibiotics in the acute inflammatory period followed by delayed surgical cholecystectomy. The perceived advantage of this approach was that the operation would be technically easier due to lack of acute inflammation. Besides the additional cost incurred with this approach, the recurrence rate of acute cholecystitis can be as high as 30 % over a 3-month waiting period [67–69]. It is important to note that in one third of these recurrences, patients presented with biliary obstruction (i.e., cholangitis and biliary pancreatitis that were more severe than the initial presentation) [67]. Elderly patients are also known to be more likely to present with complicated acute cholecystitis (i.e., gangrene, perforation, or emphysematous cholecystitis), all of which are more likely to require emergent surgical intervention with subsequently increased morbidity and mortality [26, 70].
Two recent systematic reviews of the literature compared early laparoscopic cholecystectomy (within 24–72 h) versus late operation (6–12 weeks after initial presentation) [71, 72]. Both meta-analyses found that there were no significant differences in conversion rates to open procedures, incidence of common bile duct injuries, or postoperative complications. Early laparoscopic cholecystectomy was also shown to be associated with decreased hospital lengths of stay as well as total costs [71]. However, it should be noted that in one of the meta-analysis, the incidence of bile leaks was higher in the early cholecystectomy group (3 % vs. 0 %) [71]. Also due to the small number of total patient in these pooled randomized trials (n = 451), the incidence of common duct injury could easily be over- or underrepresented in either group due to the low overall incidence of this complication (0.4–0.6 %) [73]. Despite these limitations, the consensus of both meta-analyses is that early laparoscopic surgery is safe in the hands of experienced surgeons and should be considered the preferred management strategy in patients with acute cholecystitis.
Even with these evidence-based recommendations, elderly patients have been shown to be more likely to be managed differently than younger patients. Previous studies have documented that up to 30 % of elderly patients do not have any therapeutic intervention for acute gallstone disease [74, 75]. These delays in treatment are also well documented to result in another symptomatic biliary admission (i.e., cholecystitis, cholangitis, or biliary pancreatitis) in up to 38 % of patients [76, 77]. This finding was perceived to be secondary to increased comorbidities or presentation with acute complicated disease [6, 75]. Recently, in a single-institution review, Bergman and colleagues showed that this might not be the case [70]. They found that increasing age was independently associated with a lower likelihood of surgical intervention after adjusting for severity of biliary disease as well as pre-existing medical comorbidities. Additionally, the group at Los Angeles County retrospectively compared the outcomes of elderly patients (age greater than 65) presenting with acute cholecystitis undergoing early (within 24 h of admission) vs. delayed cholecystectomy (>24 h) [78]. They found no significant differences in postoperative complications, open conversion rates, or in-hospital mortality between the two groups, while anesthesia time and hospital stays were significantly shorter in those patients that had early cholecystectomy. These findings should give surgeons pause to delaying intervention in elderly patients. Furthermore, operative delays also increase the incidence of emergency surgical intervention [74, 75] with mortality rates as high as 6–15 % [53]. This contrasts with appropriately selected elderly patients that have electively scheduled cholecystectomy and outcomes that are similar to younger patients. Conversely, patients that have a score of more than 3 on the Charlson comorbidity index have been shown to have a 2-year mortality rate of 40.4 % [15], and the PREOP-Gallstone may be helpful in the decision-making process in this patient cohort. Thus, the take-home message should be that age alone should not exclude early operative management in elderly patients presenting with acute cholecystitis.
Some elderly patients with acute cholecystitis will present with severe sepsis and septic shock or have comorbidities that are not optimized which would preclude from undergoing surgical cholecystectomy safely. These patients need to be aggressively treated with admission to the intensive care unit (ICU) and early broad-spectrum antibiotics. Obtaining source control in these patients obviously presents a clinical challenge. Two less invasive procedures, PCT and ERCP, should be considered in these patients once underlying physiologic derangements have been corrected by resuscitation.
PCT can either be done at the patient’s bedside under ultrasound guidance or in the fluoroscopy suite. After initial aspiration, a pigtail drainage catheter can be left in place for removal of further infected bile. Cultures of the bile should be sent to tailor empiric antibiotic therapy, particularly if the elderly patient has come from a nursing facility or has received recent antibiotic exposure as this is associated with a higher incidence of resistant organisms that traditional antibiotics may not cover adequately. PTC has excellent efficacy and results in the resolution of sepsis in up to 87 % of critically ill patients, with acceptable 30-day mortality rates [79]. This temporizing measure can allow optimization of the patient’s critical illness as well as any other underlying comorbidities. The drainage catheter should be left in place for 6 weeks to allow for establishment of a fibrous fistula tract prior to removal. In certain patients that are a prohibitive surgical risk due to their underlying medical problems, conservative management with PTC cholangiography with stone extraction and catheter removal can be accomplished successfully often without recurrence [61, 80]. In a long-term follow-up study with a mean duration of 3 years, 183 critically ill patients undergoing only PTC for acute cholecystitis had a recurrence rate of only 12 % [57].
ERCP with selective cannulation of the cystic duct and stent placement is another treatment modality that may be particularly useful in critically ill patients that are unable to undergo PCT particularly in the setting of coagulopathy or uncontrolled ascites [81].This procedure is more technically challenging than ERCP and requires advanced endoscopy skills. In experienced hands, this procedure is successful in over 90 % of cases with a reported clinical efficacy of 80–90 % [81].
It should be stressed to the reader that PCT should only be used as a treatment modality in those patients that are too critically ill or medically unfit to undergo a surgical operation as this procedure is associated with increased hospital lengths of stay and up to a 25 % rate of readmission for biliary-related complications. A detailed Cochrane review comparing PCT versus cholecystectomy as initial treatment for severe acute cholecystitis found no evidence to support the use of PCT over surgical intervention [82]. A recent large retrospective single-institution review reached similar conclusions even when accounting for patients that underwent conversion to open cholecystectomy [55]. Those patients that were treated surgically had shorter lengths of stay, as well as a lower number of complications and readmissions compared with those that underwent PCT as treatment for acute cholecystitis. Only those patients that presented with increased comorbidities and medical risk for surgery as defined by the Charlson comorbidity index appeared to benefit from PCT over surgical intervention for acute cholecystitis [55]. Despite this preponderance of evidence, a recent national review by Duszak and Behrman documents a 67 % increase in the use of PCT over the last two decades [83].
Chronic Calculous Cholecystitis
Clinical Presentation and Diagnosis
Chronic cholecystitis is typically the most common manifestation of symptomatic calculous disease and occurs in the setting of multiple (and often insidious) episodes of biliary colic. This scenario is typical in the elderly due to the alterations in pain perception and immune response to inflammation that have been described (see section “Diagnostic Investigation”). The patient often presents with symptoms similar to those of acute cholecystitis without systemic signs of inflammation. Typically the pain is located in the epigastrium/right upper quadrant and is dull or of less intensity due to the absence of acute peritoneal irritation. The patient’s temperature, white blood cell count, and liver function tests are most commonly within normal limits. The metabolic panel should also be checked, particularly in the elderly patient, as they often are on medications that can cause fluid and electrolyte derangement. Ultrasonography again should be the initial imaging test of choice that often demonstrates stones within a thickened contracted gallbladder wall. The “double arc” sign is pathognomonic for this condition when seen on ultrasonography [32]. Occasionally chronic cholecystitis will be associated with mural calcification of the gallbladder wall. This may involve a portion of the wall or the entire gallbladder otherwise known as a “porcelain” gallbladder (Fig. 14.10). The significance of this finding has been controversial due to the potential association with gallbladder carcinoma particularly in the elderly patient [84–86]. Ultrasound findings that are suggestive of this pattern and that show loss of delineation between the gallbladder wall and liver parenchyma where there is no serosal layer should lead one to consider additional diagnostic imaging such as CT or MRI [87].
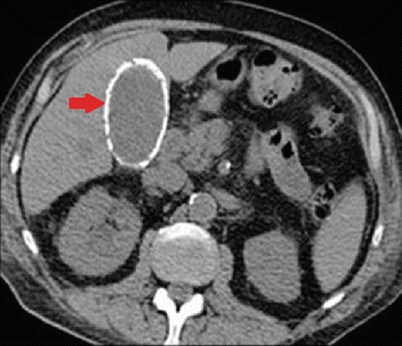

Fig. 14.10
CAT scan of the abdomen showing complete calcification of the gallbladder wall (arrow) consistent with a “porcelain” gallbladder
Treatment of Chronic Calculous Cholecystitis
Once chronic cholecystitis has been diagnosed, initial treatment should be directed at relieving pain symptoms with the judicious use of narcotics. Intravenous fluids should be given as the patient often will be dehydrated from diminished oral intake and may need electrolyte correction. After acute symptoms have resolved, the patient should be risk stratified for surgery. If patients are acceptable candidates, elective laparoscopic cholecystectomy should be undertaken as the natural history of chronic cholecystitis is to recur (40 % chance over 2 years) [88]. Delaying operation because of age and waiting for an episode of recurrence of disease in the elderly are hazardous as they often present in a delayed fashion and with a higher incidence of complicated calculous disease that may require urgent as opposed to elective surgical intervention that is associated with higher morbidity and mortality [26, 53, 70, 88]. Elderly patients may also be considered candidates for same-day discharge or ambulatory cholecystectomy in certain instances, with success rates of 70 %, or more documented in the literature [89].
A special form of chronic cholecystitis that deserves mention is the “porcelain gallbladder.” Though the incidence of porcelain gallbladder remains low (0.2 % in a recent large series) [61], there is concern of the potential for malignancy, particularly in those over 50 years of age (incidence of 0.08 %/year of symptoms) [88]. The management of the porcelain gallbladder has undergone considerable change over the past several decades. Early reports suggested a high association between porcelain gallbladder and gallbladder carcinoma (up to 60 %) [90] which led to recommendation of open cholecystectomy once the diagnosis was made. Recently, several large clinical series have questioned the significance of the porcelain gallbladder after finding a much lower incidence of malignancy (0–5 %) [85, 86, 90]. The reasons for this dramatic shift are felt to be due to advances and increased usage of abdominal imaging as most cases of porcelain gallbladder were only diagnosed on plain films of the abdomen, geographic variation of study, and wider usage of laparoscopic cholecystectomy [90].
The overwhelming majority of elderly patients that have a porcelain gallbladder identified on imaging have symptomatic disease which would make them surgical candidates unless their preoperative estimated risk was found to be prohibitive [85, 86, 90]. The asymptomatic patient with incidental findings of a porcelain gallbladder represents a clinical impasse on whether to proceed with operative intervention or observe the patient. The risk of surgical intervention, particularly in the patient with comorbidities, should be balanced against the low potential risk of gallbladder carcinoma and discussed with the patient in order to establish a course of action. Laparoscopic cholecystectomy has been found to be technically feasible in patients with a porcelain gallbladder and should be the initial procedure of choice with more aggressive intervention being reserved for those patients that are found to have cancer on their final pathology or intraoperative findings that are suggestive of carcinoma [90, 91].
Choledocholithiasis
Clinical Presentation and Diagnosis
The majority of common bile duct stones that lead to symptoms originate from the gallbladder itself and can lead to a wide array of clinical symptoms. The elderly population also has a higher incidence of common bile duct stones (range 15–20 %) that present with symptomatic calculous disease compared to younger patients [53]. Up to a third of common duct stones will spontaneously pass into the duodenum [92], while others may lead to common duct obstruction resulting in biliary pancreatitis or cholangitis.
Right upper quadrant pain with abnormal liver function tests is present in over 75 % of patients [93]. The liver panel usually shows a cholestatic (elevated serum bilirubin) pattern, and transaminases may be >500 IU/L along with elevation of alkaline phosphatase or serum gamma-glutamyl transferase (90 % of cases) [94]. Leukocytosis may also be present in the acute phase, and coagulation parameters should be routinely checked as biliary obstruction can lead to transient vitamin K deficiency and subsequent coagulopathy. It should be noted that around 10 % of patients will be asymptomatic with only mildly elevated liver function tests and common duct stones that are found incidentally on imaging for a reason other than biliary symptoms [93, 95]. In the elderly, malaise, altered mental status, or acute deconditioning may be the only presenting symptoms [62].
Abdominal ultrasonography should be the initial imaging test of choice and has excellent sensitivity for detecting biliary ductal dilation (see Imaging section) and may directly visualize common duct stones. If the ultrasound is normal but clinical and laboratory testing is suggestive of choledocholithiasis, then MRCP should be considered as this has higher sensitivity than sonography. ERCP should generally be reserved for therapeutic purposes due to its invasive risks and technical complications.
Treatment of Choledocholithiasis
Once the diagnosis of choledocholithiasis is made, there are a variety of treatment options available, and these should be tailored based upon local expertise and resource availability. Initial treatment should be directed at alleviating pain, fluid resuscitation, and correction of any electrolyte or coagulation disorders that may be present. Complete removal of common duct stones should be the objective regardless of the intervention chosen because up to 50 % of patients will have recurrence of symptoms if left untreated, and 25 % of these recurrent cases will result in potentially serious complications (i.e., biliary pancreatitis or cholangitis) [94].
Endoscopic therapy with ERCP or percutaneous intervention (PTC) are both acceptable methods of ductal clearance according to the Society for Surgery of the Alimentary Tract (SAGES) and British Society of Gastroenterology guidelines [39, 52] and should be chosen based upon local availability and expertise. As elderly patients are often on anticoagulants or antiplatelet medications, these should be withheld in anticipation of therapeutic intervention. Antibiotics are also typically given periprocedurally and should again be directed primarily against the Enterobacteriaceae family. ERCP with balloon dilation of the sphincter has an excellent clinical success rate and appears to be safe even in the elderly population with known comorbidities [40, 53].
Once ductal clearance has been achieved, elderly patients should be offered cholecystectomy if they are acceptable surgical candidates because of the potential for recurrent biliary symptoms. In a 2-year prospective investigation, Lee et al. found that the age and the presence of comorbid conditions were risk factors for recurrence of choledocholithiasis [96]. Additionally, these patients were felt to be at higher surgical risk at the time of recurrent presentation than if they had cholecystectomy at the time of initial presentation. Similar findings were seen in a large systematic review done by the Cochrane group which found a decreased recurrence rate and increased survival advantage even in patients deemed “high risk” were managed with early cholecystectomy as opposed to a “wait and see” approach [97].
The timing of cholecystectomy after ERCP and sphincterotomy has also been investigated prospectively. In a randomized trial comparing “early” laparoscopic cholecystectomy (within 72 h) versus delayed cholecystectomy (6–8 weeks after ERCP), there were no differences in operative duration or rate of conversion to open procedures, while 36 % of patients in the delayed group developed recurrent biliary symptoms [98]. The authors concluded that early cholecystectomy prevented future symptoms related to common duct stones without an increase in morbidity in those undergoing early operation. Patients that undergo ERCP with sphincterotomy and ductal clearance are still at risk for residual choledocholithiasis. Clinical studies assessing this risk in prospective series have found it to be as high as 13 % [99, 100]. Therefore, the authors recommend routine intraoperative cholangiography in patients that had a preoperative ERCP with ductal clearance to reduce the chance of having retained common duct stones (Fig. 14.11). For those surgeons with advanced laparoscopic skills and institutions that have the instrumentation and imaging capability, single-stage procedures to treat choledocholithiasis include laparoscopic cholecystectomy with intraoperative cholangiogram followed by common bile duct exploration have gained popularity. This leads to decreased hospital lengths of stay and total charges without an increase in associated morbidity or mortality [101–103]. This operative approach has also been validated in the elderly and high-risk patients [104]. The benefits of one-stage treatment of choledocholithiasis have only been observed in uncomplicated cases (i.e., no cirrhosis, cholangitis, or biliary sepsis) [101] and with adequate laparoscopic experience [105].
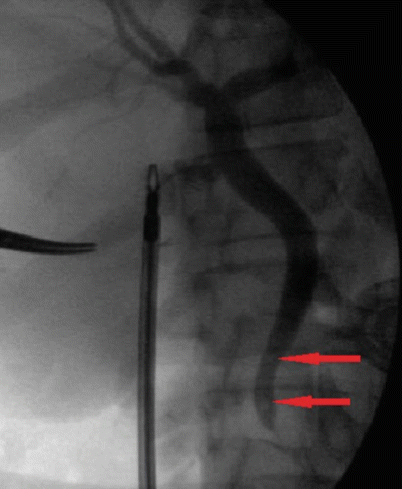

Fig. 14.11
An intraoperative cholangiogram demonstrating two filling defects consistent with common bile duct stones (arrows)
Cholangitis
Clinical Presentation and Diagnosis
Cholangitis can follow a wide spectrum of disease in the elderly patient, from a mild infection to fulminant septic shock with multiple organ dysfunction. Early recognition of which form of disease is present is imperative to achieve good clinical outcomes. An obstructing common duct stone is the most common etiology of cholangitis but may also occur in the presence of benign or malignant biliary strictures. Stasis of bile leads to bacterial overgrowth from the duodenum with Escherichia coli being the most common offending organism followed by other members of the family Enterobacteriaceae. Increased amounts of pressure in the biliary tree lead to translocation of bacteria into the bloodstream with resultant toxemia [94]. The classic clinical picture of Charcot’s triad (fever, right upper quadrant pain, and jaundice) is present in 70 % of patients [94]. The addition of altered mental status changes and hypotension to Charcot’s triad form Reynold’s pentad and are indicative of suppurative cholangitis, a surgical emergency. In addition to clinical exam findings, laboratory studies often reveal leukocytosis with elevated liver transaminases and cholestasis. Thrombocytopenia, decreased serum bicarbonate (reflective of metabolic acidosis), and elevation of creatinine are diagnostic of more severe cholangitis [106]. Coagulation studies should also be checked in preparation for ductal decompression as biliary obstruction with superimposed sepsis can lead to coagulopathy. Diagnostic imaging is frequently done with ultrasonography that typically reveals dilation of the biliary tree.
Treatment of Cholangitis
Initial therapy should be to establish intravenous access and begin fluid resuscitation followed by early administration of broad-spectrum antibiotics. Appropriate initial antibiotics include third-generation cephalosporins, fluoroquinolones combined with metronidazole, or beta-lactamase inhibitor combinations (i.e., piperacillin/tazobactam) [63]. Blood cultures should be obtained but not delay antibiotic therapy. Since most patients present with mild-moderate cholangitis (nonsuppurative), this will lead to clinical improvement in anticipation of ductal decompression within the next 24 h [52]. Even with initial improvement, elderly patients should be closely monitored for decompensation since age (>50 years) has been shown to be a predictor of poor outcomes [107]. Once sepsis has resolved and the patient is otherwise medically fit, laparoscopic cholecystectomy should be offered to the patient.
For those patients that present with florid sepsis and organ dysfunction, they should be aggressively resuscitated and transferred to the intensive care unit for invasive monitoring. Hemodynamic support with vasopressors may be required in cases of septic shock, and optimization of physiologic parameters should be undertaken to allow for source control through biliary decompression through the least invasive means available. Endoscopic decompression with ERCP has become the initial procedure of choice for most elderly patients [94]. The aim of the procedure is to relieve biliary pressure through minimal manipulation to prevent exacerbation of endotoxemia. Sphincterotomy with biliary stent placement is the most frequently utilized technique. Should ERCP be technically difficult and unsuccessful, external decompression using PTC is a good second-line option. Even with successful decompression, there is still mortality of 5–10 % [108]. In situations where ERCP/PTC is unsuccessful or unavailable, drainage can be accomplished surgically with open common bile duct exploration and T-tube placement. The mortality for this intervention is much higher than the preferred nonsurgical techniques (16–40 %) [93]. After successful biliary decompression and stabilization of the patient, antibiotic therapy should be continued for 14 days and the patient risk stratified for cholecystectomy once optimized from the medical point of view.
Biliary Pancreatitis
Clinical Presentation and Diagnosis
In some cases of choledocholithiasis, the stone transiently lodges at the ampulla and causes an obstruction of the pancreatic duct. This leads to intraductal activation of enzymes and pancreatic glandular damage with a generalized inflammatory response that leads to subsequent symptoms. Risk factors for the development of biliary pancreatitis include advanced age (>60) and female gender [109]. Most cases of biliary pancreatitis are moderate and will resolve with supportive care. However, severe cases of pancreatitis can lead to sepsis and multiple organ dysfunction with mortality rates that exceed 20 % [110] and require a multidisciplinary approach with critical care support.
Clinical symptoms of acute biliary pancreatitis include sharp epigastric pain with radiation to the back that can be confused with aortic dissection or myocardial ischemia in the elderly. Nausea and vomiting are also typically present. Signs of systemic inflammation such as tachycardia and fever may also be present. In severe cases, the patient can also have hypotension and altered mental status. Helpful laboratory investigations include CBC, serum chemistry with a serum calcium level, liver function tests, and serum amylase and lipase. Leukocytosis is often present secondary to inflammatory response, and the hematocrit is often elevated from hemoconcentration. Serum chemistry can also depict signs of impaired tissue perfusion if the serum bicarbonate is low or renal parameters are indicative of acute renal impairment. Transaminases are often greater than 500 IU/L in the acute phase with a moderate elevation of serum bilirubin. Typically both the serum amylase and lipase will be elevated, but serum amylase declines earlier in the time course of pancreatitis and may be normal in cases of delayed presentation.
Once the diagnosis of biliary pancreatitis is suspected, the initial imaging test should be an abdominal ultrasound. This test can be done even in unstable patients as it can be done at the patient’s bedside. Ultrasound is diagnostic of biliary pancreatitis if it reveals calculi or sludge in the gallbladder. Dilation of the biliary tree may also be noted. In patients that are clinically unstable or that do not improve with resuscitation, CT imaging can be helpful in assessing the severity of pancreatitis [111]. The initial study should be non-contrast as it is still clinically useful and avoids exposing a hypovolemic elderly patient to a potentially nephrotoxic contrast load. A CT scan with IV contrast can be obtained at a later time when the patient is clinically stable to delineate areas of potential pancreatic necrosis.
Treatment of Biliary Pancreatitis
Initial therapy is directed at alleviating pain with judicious use of narcotics and nasogastric decompression for those patients presenting with symptoms of ileus. Generous fluid resuscitation should also be undertaken due to the amount of fluid sequestration that can occur in the retroperitoneum. There should be a low threshold to admit elderly patients to the ICU even in moderate cases due to their limited physiologic reserve. Many different scoring systems for determining the severity of pancreatitis have been developed [112–115], but the Ranson score has been shown to have the highest predictive accuracy [113]. A Ranson score of 3 or more is indicative of severe disease. Well-established evidence-based guidelines have been developed for treatment [116, 117].
There are three areas of current debate regarding the optimal therapy of biliary pancreatitis. The first area of controversy in the treatment of severe pancreatitis is the use of prophylactic antibiotics. Several systematic reviews examining the utility of antibiotic prophylaxis in severe acute pancreatitis have been undertaken and have failed to demonstrate any protective benefit on mortality or the risk of developing infected pancreatic necrosis [118–120]. Due to problems with emerging bacterial resistance, the authors recommend against the routine use of antibiotic administration in sterile cases of severe pancreatitis. In instances when superimposed cholangitis is suspected with biliary pancreatitis, appropriate antibiotic therapy is warranted.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





