(1)
Northern Anesthesia & Pain Medicine, LLC, Eagle River, Alaska, USA
(2)
WWAMI Program, University of Washington School of Medicine, Anchorage, Alaska, USA
Keywords
OpioidsPreemptive analgesiaMultimodalChronic postsurgical painChronic non-cancer painNeuropathicVisceralCancerEvidence-based medicineRandomized controlled trialSystematic reviewMeta-analysisDyspneaA 62-year-old female patient is referred to you by a colorectal surgeon in consultation regarding pain management for advanced (Stage IV) colorectal adenocarcinoma that has involved the majority of her lower pelvis, with metastases to the liver and brain. She complains of severe deep pelvic pain as well as perineal pain exacerbated by sitting, and dyschezia/tenesmus. Secondarily she also complains of lower segment posterior neck and shoulder girdle pain without radicular symptoms, and with a cramping/aching quality, for which she has found cyclobenzaprine to be moderately effective in relieving. Her past medical history is otherwise notable for hyperthyroidism and psoriatic arthritis. She has a remote history of opioid dependence as well and is very concerned about the referral to you, as she “doesn’t want to be addicted again.” She has however been using Percocet 10 mg/325 mg every 2–3 h. Scintigraphy shows no concerning osseous lesions; her liver function studies are within normal limits.
Together you and her decide on a pain management plan that includes:
- 1.
Meloxicam 7.5 mg qd.
- 2.
Gabapentin titrating up to effect from 300 mg qhs.
- 3.
Fentanyl 37 mcg/h. Patch with hydromorphone 2 mg tablets q4 h PRN after explaining to her that in the context of terminal cancer with severe pain, opioid therapy is considered beneficial to not only improve quality of life but also potentially reduce pain-related immunosuppression.
- 4.
Venlafaxine beginning in 2 weeks after assessing her response to the previous interventions. You explain to her that you don’t want to start too many new agents simultaneously, so as to avoid confounding potential adverse effects.
- 5.
Pudendal nerve and ganglion impar neurolysis, with possible superior and inferior hypogastric plexus neurolysis depending on her response to the former.
- 6.
Referral for myofascial release/manual therapy for her cervicalgia/shoulder girdle pain.
Introduction
Opioids remain a prevalent therapeutic modality for a reason: They possess significant analgesic effects across a wide spectrum of painful conditions. Often referred to as “broad-spectrum analgesics ,” they reduce nociception and perception and enhance descending modulation with time-tested efficacy. Mounting evidence however suggests that as with most tools within medicine, there is a time and place for opioid therapy, and “blanket use” to treat pain of all shapes and sizes, for an indiscriminate duration, may be detrimental. Tolerance with resultant decrease in the benefit-risk ratio, adverse effects including hyperalgesia, and simple inefficacy in many situations mandate that the physician knows why and when to prescribe opioids (the subject of this chapter) and perhaps more importantly, when not to (Chap. 8 is devoted to how).
So why should the clinician prescribe opioids for pain? The first and most obvious reason is to relieve severe pain that cannot be relieved otherwise, assuming the risks do not outweigh the benefit. A well-established and honored guideline as discussed in more detail below and in the next chapter is to reserve the use of opioids for:
As introduced in the previous chapter, however, pain is a subjective and individual experience with heterogeneous (and generally multifactorial) etiology. Fatigue is similarly subjective and individualized and also shares a plethora of potential causes. Universally prescribing thyroid hormone replacement to all fatigued patients is inconceivable. Hypertension similarly has different etiologies (e.g., vascular, renal, cardiac, neuroendocrine factors) that contribute to varying degrees in any given individual, and while profound vasodilators such as sodium nitroprusside or phentolamine will effectively lower anyone’s blood pressure, this may be not only overkill but literally lethal.
Intractable … pain that is not adequately managed with more conservative or interventional methods. [1]
Granted, fatigue and hypertension do not share the same current public nor professional conviction regarding a “moral imperative ” [2] for treatment that pain does, nor are they generally associated with the same degree of distress and emotional urgency experienced and communicated by most patients in pain. This is not intended in any way to make light of the pain and suffering of any individual. It is intended rather to highlight the fact that while it is second nature for physicians to approach fatigue or hypertension (or most clinical issues) with an analytical mindset, taking a good history and physical and ordering appropriate tests, and tailoring (hopefully evidence-based) therapy to that individual, the same is rarely true of dealing with patients in pain. Pain often brings with it a sense of urgency that can supersede the ration of both patient and physician, and these pressures are arguably greater upon the uninformed and untrained provider. A better understanding of the nature of pain was the intent of the previous chapter, and a better understanding of the efficacy (or lack) of opioids in treating pain is the thrust of this one.
The second reason—perhaps goal is a better word—for a physician to prescribe opioids, and one that has been highlighted recently in several consensus opinions and guidelines [1, 3–5], is to facilitate functional improvements in people who would otherwise be unable to achieve such improvement. This subject is addressed in greater detail in the next chapter.
Opioid prescribing patterns have historically swung in pendulum fashion between conservative and liberal practice, with competing societal concerns for personal and community damage versus inadequate treatment of pain and suffering. Lack of consensus opinion on any topic generally indicates incomplete collective knowledge and understanding of a subject, and this is arguably true of medicine in general. An often-quoted statistic is that roughly half of what we believe to be true isn’t. Constant evaluation of past and present (as well as what the near future may hold) is necessary to offer sound advice and treatment to those who put their trust in us. As such, and in keeping with the current climate of evidence-based medicine , this chapter will attempt to present in greater detail the why and when (with some when nots, e.g., headache, fibromyalgia, etc.) of opioid prescribing for various scenarios, including:
within the context of recent literature review on therapeutic efficacy or lack thereof.
Acute pain, including perioperative pain
Chronic “inflammatory” pain
Chronic “neuropathic” pain
Chronic visceral pain
Cancer pain
Opioid Therapy in Acute Pain
General Considerations
When should the physician (or if within legally defined scope of practice, the mid-level practitioner) prescribe opioids for acute pain ? The relief of pain and suffering has always been recognized as a fundamental moral and ethical responsibility of the physician (within the confines of positive benefit-risk ratio, standards of care, legality, etc.) It has long been appreciated in acute care settings such as the emergency department and also in operative medicine/anesthesia that severe untreated pain may have highly negative physiologic consequences including hypertensive crises and their sequelae, myocardial ischemia due to demand/supply imbalance, etc. Quality of life, functionality, and overall well-being are also affected both in the short term and frequently long term, depending on how well the acute pain is treated [6, 7]. There is also evidence that how acute pain is managed frequently dictates whether or not the painful condition becomes chronic [7, 8], and our growing understanding of long-term neuronal potentiation and plasticity and sensitization provides a biologic rationale for effective acute pain relief whenever possible. Long-standing strategies such as preemptive analgesia (discussed in greater detail below) to prevent chronic postsurgical pain states [9] or immediate antiviral treatment of shingles to prevent post-herpetic neuralgia [10] illustrate the widespread utility of this principle (Fig. 7.1).
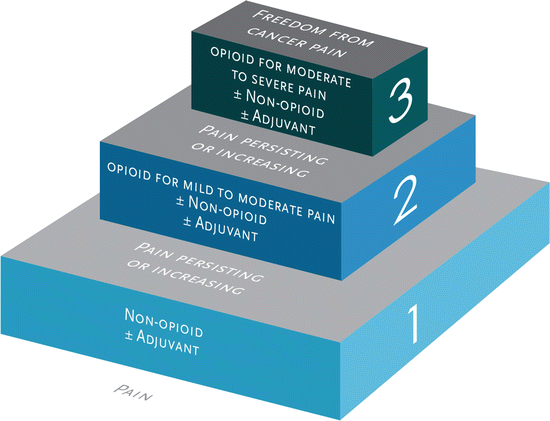

Fig. 7.1
World Health Organization analgesic ladder
The use of opioids to treat severe acute pain with clearly demonstrable physical pathology is rarely contested (although application of a stepwise approach, e.g., the WHO ladder [see Figure 7.1], with opioid use reserved for severe and refractory pain, is certainly consistent with good practice of balancing risks, benefits, and alternatives). This stepwise approach, and the associated multimodal analgesic strategy, has been adopted by numerous organizations and federal agencies including the American Society of Anesthesiologists [11], the American Academy of Pain Management [12], the Washington State Agency Medical Directors’ Group [3], the Institute for Clinical Systems Improvement [13], the US Military Joint Theater Trauma System [14], the Veterans Administration [15], and many others. Given what we know about the pathophysiology of pain (including the tremendous contributions of psychosocial-spiritual factors), there are ample opportunities for intervention upon acute pain including reductions in nociception and peripheral sensitization (e.g., cooling modalities, anti-inflammatories), transmission (e.g., local anesthetics, anticonvulsants), and perception and modulation (e.g., brief behavioral interventions and counseling/education, anxiolytics/sedatives, opioids). Such multimodal care not only improves analgesia but also reduces the risks of adverse effects of any one therapeutic class and in particular opioids.
The use of opioids to treat acute pain must also be carefully analyzed in light of comorbidities including respiratory, gastrointestinal (e.g., ileus, pancreatitis), renal and psychiatric pathology. Comorbidities aside, evidence also exists to support the limitation of opioids in certain situations that might otherwise seem innocuous, such as acute low back pain; the prescription of opioids for acute low back pain for greater than 1–2 weeks has been shown to predict greater loss of function and long-term disability [16, 17].
“Acute on chronic” pain occurring in the chronic pain patient, and especially the chronic pain patient on chronic opioid therapy (COT ), is an exceptionally difficult situation to intervene upon to the satisfaction of both patient and provider [18, 19]. Such so-called breakthrough pain (borrowing from the oncologic lexicon) may in fact be better treated in many cases with a combination of basic injury treatment (e.g., rest, ice, elevation), non-opioid pharmacotherapeutics, brief counseling and anxiolytic techniques (e.g., diaphragmatic breathing, progressive muscle relaxation, and guided imagery), and other soothing activities (e.g., warm baths, massage, etc.)
In summary, opioid therapy has a time and place in the treatment of acute pain but only in the context of a multimodal approach, and with great care exercised toward the prevention of dependence, with a clearly delineated “exit strategy” (discussed in greater detail in Chap. 8).
Perioperative Opioid Therapy
A special subset of acute pain deserving unique consideration is perioperative pain, the majority of which is associated with elective procedures that can be anticipated and planned for. Perioperative pain (POP ) is virtually universal, and the literature consistently reports a prevalence of chronic postsurgical pain (CPSP) in the neighborhood of 50%. Neuropathic pain and central sensitization are believed to represent a significant component of most CPSP states [20–24]. Increased POP intensity/poor (immediate) postsurgical pain control has been understood to be a risk factor for CPSP for decades and confirmed recently by robust investigations [25–27]. Increased POP also shares psychological confounders which are strong independent predictors of increased CPSP [26–31].
Opioids have enjoyed perioperative (including pre- and intraoperative) use for over 150 years in conjunction with sedative-hypnotic agents to facilitate surgery as well as relieve some of the severe associated pain. The concept of “balanced anesthesia ” taught to every anesthesiologist in their training includes (generally intravenous, intraoperative) opioids within the admixture, as these agents have historically provided unparalleled efficacy in analgesia and sympatholysis, with widespread availability and low cost. The concept of preemptive analgesia (PEA) was first proposed by Dr. Patrick Wall (of gate theory fame, as discussed in the previous chapter) [32] and later developed more fully by his protégé Dr. Clifford Woolf, who demonstrated that prevention of central sensitization underlies the mechanism of PEA [33]. In essence the theory states that adequate analgesia surrounding a traumatic insult (e.g., surgery) can prevent the chronification and amplification too often seen with a surgical operation. Initial interpretation and application of PEA were confined to the preoperative (including immediate pre-incision moment) period and focused on blunting or eliminating nociceptive input by means of systemic analgesics (e.g., intravenous opioids or ketamine) and/or local anesthetic. Despite widespread enthusiasm for the concept, the data did not support the efficacy of this (exclusively pre-incisional) strategy until expanded awareness of the ongoing contribution of low-level C-fiber transmission from the surgical wound is critical in maintaining the sensitized state, necessitating ongoing analgesia until adequate healing occurs [33]. Opioids have continued to represent the lion’s share of PEA practice, as ongoing adequate local anesthetic blockade is technically challenging and logistically difficult and prolonged ketamine use (or rather cumulative excess) confers significant dissociation and psychosis. However, it has been recognized for quite some time, well prior to the onset of the current opioid epidemic, that:
Increasing awareness of the importance of the perioperative arena as a significant contributor to the opioid epidemic has focused research on elucidating risk factors for prolonged postoperative opioid use (PPOU ) [34]. Over 100 million surgical procedures are performed in the United States every year at present [35], and the prevalence of long-term opioid use in previously opioid-naïve patients following surgery appears to be on the order of 1:20–1:15 patients [36, 37]. This yields a rough incidence of 50,000 new individuals per year using chronic opioids. (The prevalence of chronic postoperative opioid use in opioid-experienced patients is substantially higher.)
Until an opioid without side effects is available, opioid sparing strategies need to be adopted to ensure sufficient analgesia without sedation and nausea…. [33]
The Toronto General Hospital Transitional Pain Service investigated risk factors for PPOU with a retrospective study of nearly 40,000 opioid-naïve Canadian patients undergoing major elective operations between 2003 and 2010 [38]. Patient-specific (not associated with type of operation or medical comorbidities including diabetes mellitus, congestive heart failure, or pulmonary disease) risk factors included younger age and lower household income. Preoperative use of benzodiazepines and antidepressants, as well as angiotensin-converting enzyme inhibitors, was also independently associated with PPOU.
Recent work done by the Stanford University Anesthesia and Pain Medicine Department has identified preoperative depression, self-perceived addiction vulnerability, and preoperative opioid use as risk factors for PPOU [39, 40]. An investigation of orthopedic trauma patients at the Massachusetts General Hospital also identified catastrophic thinking patterns, anxiety, depression, and post-traumatic stress disorder (PTSD) as predictors of prolonged (greater than 1–2 months) postoperative opioid use, with the former holding the strongest association [41]. A recent study done in a Veterans Administration patient population also reported PTSD as a risk factor for PPOU [42].
Multiple investigations have shown, not surprisingly, that preoperative use of opioids, whether licit or illicit, confers a high risk of PPOU [26, 39–41, 43–45]. Preoperative opioid use has also been universally documented (and dreaded by providers and nursing staff) as a poor predictor of adequate/satisfactory perioperative analgesia as well as patient safety; increasing awareness of the complexities of dealing with such “acute on chronic” pain may fortunately be driving practice toward improved preoperative assessment and a multimodal approach to perioperative analgesia [46–49], the latter of which was discussed in the previous chapter. (Also of interest to patients, surgeons, and hospitals alike should be the additional findings that preoperative opioid use is associated with worsened functional outcomes, decreased satisfaction with the operation, and increased length of stay/increased pain management referrals postoperatively [44, 50–52].)
Surgeons, anesthesiologists, and pain physicians all have an opportunity within this critical window to improve the health of their individual patients as well as institute more generalized strategies impacting the epidemic. Screening for patients at high risk of PPOU should be a standard part of the preoperative assessment [53] and discussed candidly with the patient. Given the strong correlation between psychodysfunctional diathesis and both increased CPSP and PPOU, it may be in the best interest of all parties involved, including the taxpayer, to explore on a large scale whether preoperative psychological clearance prior to elective operations yields improved outcomes. Such an approach has been routine for many years now for certain procedures such as spinal cord (dorsal column) stimulator implantation and bariatric operations. A more targeted approach, given the apparent associations between preoperative opioid use and depression, anxiety, PTSD, etc., using simple screening methods such as state-run prescription database monitoring systems (discussed in greater detail in the next chapter) to risk stratify patients into groups dictating the salience of preoperative psychological assessment and intervention may be “higher yield.”
Second, increasing awareness of the paramount contributions of anxiety and catastrophization to CPSP mandates that education on expected perioperative pain/discomfort (which has been shown for over half a century to be beneficial [54, 55]) must continue to be emphasized and practiced by surgeons and their care teams. Consultation of behavioral health professionals for additional preoperative assistance with overcoming cognitive distortions may be uniquely effective [56].
Third, the use of non-opioid modalities to reduce POP has been advocated by anesthesiologists for over a quarter of a century. A significant practice shift among anesthesiologists and surgeons has occurred even within the past decade; whereas opioids have historically comprised the major, if not sole, pre-, intra-, and postoperative analgesics, they are increasingly viewed as “rescue agents” with numerous non-opioid “first-line” or at least adjunctive alternatives [57, 58] receiving greater attention and utility, as discussed in the previous chapter.
Opioid Therapy in Chronic Non-cancer Pain
The use of prescription opioids in chronic non-cancer pain (CNCP) was rare in the past century until the mid-1990s, when a constellation of factors including pressure from the parascientific community (ranging from the American Pain Society and Joint Commission to the Federation of State Medical Boards), aggressive marketing by pharmaceutical companies, lawsuits against physicians alleging inadequate analgesic prescribing, and increasingly emboldened requests from patients appear to have changed the “standard of care” in America. Dovetailing with this latter factor is the subtle leverage exerted upon many hospital-based physicians over the past decade by the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey administered to patients upon discharge. Within the HCAHPS survey are questions on patient satisfaction related to pain management during their hospitalization, and as the Centers for Medicare and Medicaid Services (CMS) bases facility reimbursements in part on this survey, it has been argued recently that physician opioid-prescribing behavior has been influenced toward more liberal patterns by these incentives (or fear of sanction). CMS has recently announced its intent to remove the survey’s pain management questions from its calculations of hospital reimbursement [59]. In the outpatient arena, physicians face similar pressures from burgeoning internet reviews, satisfaction ratings, etc. that can have tremendous impact on small practice viability [60].
Regardless of the causal factors involved, the past 20 years have seen an unprecedented degree of liberal prescribing of opioids for non-indicated complaints of all sorts; guilty parties include not only “pill-mills” but also well-meaning but poorly informed or equipped providers trying to please patients. In the author’s opinion, a substantial contributor to the increase in patient requests for/provider compliance with opioid prescribing has been a widespread and complex breakdown of societal structure as a whole coupled with poorly reimbursed/funded/staffed behavioral health resources. Opioids are frequently requested (and prescribed) for psychological distress, and opioid dependence once established cements the pattern.
Reactionary attention toward an instruction on the topic of opioid use for CNCP has been commensurate with the magnitude of the societal issue. More detailed examination of these issues follows in the next chapter; for now, suffice it to say that it is once again becoming the general consensus within medicine at the midpoint of this decade that opioids for chronic non-cancer pain should be prescribed only:
For moderate to severe pain with a plausible etiology that is recalcitrant to other forms of treatment
When potential benefits outweigh the risks
As part of a comprehensive treatment plan involving other primary modalities as applicable, with opioids as the adjunctive therapy
With constant evaluation of benefit in terms of improved function vs. adverse effects
With clear instructions as to patient responsibility for compliance, safe use, storage, non-diversion, etc.
With exit strategy clearly delineated a priori
Objective measurement of pain is difficult at best, given that pain by definition is a subjective experience. In the operative and ICU environments, anesthesiologists and other intensivists use hemodynamic variables (or if neuromuscular function is not chemically paralyzed, respiratory effort and somatic movement) as surrogates for pain and discomfort. Postanesthesia care unit nurses learn very quickly to assess respiratory rate and pupil diameter when caring for recovering patients who are requesting more opioid analgesics (as well as assessing the patient for other potential causes of pain and discomfort such as a full bladder or rapidly expanding postoperative hematoma). Beyond these physiologic surrogates, more modern parameters such as biomarkers (e.g., cortisol levels) and biopotentials (e.g., electromyographic or electroencephalographic data) have been proposed [61] as means to help “measure” pain; at present such efforts are impractical and not cost-efficient. Near-infrared spectroscopy (a technology similar to pulse oximetry) can be used to analyze neuronal activity by means of cerebral blood flow and volume and may show promise as a noninvasive and efficient means of providing objective data.
Chronic non-cancer pain (CNCP) comprises a heterogeneous group of conditions, potentially sharing the common ground of central sensitization, but nonetheless with their own unique pathophysiology deserving consideration. In this chapter we will consider three separate entities of chronic “nociceptive” pain, neuropathic pain, and visceral pain.
Chronic “Nociceptive” Pain
Despite the suggestion that all chronic pain states are neuropathic by means of CNS alterations including central sensitization, in this section we will label traditionally understood “non-neuropathic” states as “nociceptive,” in line with the traditional viewpoint that most such conditions (e.g., musculoskeletal pain) are a peripheral issue maintained by ongoing tissue insult with associated inflammation. This category, including chronic neck and low back pain, and appendicular orthopedic and rheumatologic disease states comprise the majority of chronic pain within the developed world. As mentioned above in the introduction to this section, the use of chronic opioid therapy for such chronic pain complaints was exceedingly rare prior to the last decade of the previous millennium, with a dramatic increase in liberal use over the past 20 years or so. The resulting crisis has encouraged considerable investigation into the efficacy (or lack thereof) of chronic opioid therapy in CNCP, and the results of most of these investigations are summarized below. Reviews focusing on neuropathic pain are presented in the section that follows.
In 2003, Chou et al. [62] published what is likely the first systematic review of opioid studies. This analysis specifically examined long-acting opioids in CNCP and evaluated 24 studies including 16 randomized clinical trials (RCTs). The review focused on whether long-acting opioids showed any benefit compared to short-acting opioids, in terms of pain relief, and also compared long-acting opioids against others in that class. Long-acting codeine, morphine, oxycodone, and fentanyl were represented in the studies; methadone and levorphanol were not. The study populations included musculoskeletal and neuropathic pain . No trial extended beyond 4 months. The authors concluded that there was insufficient evidence to prove that different long-acting opioids are associated with different efficacy or safety profiles. There was also insufficient evidence to determine whether long-acting opioids as a class are more effective or safer than short-acting opioids.
Kalso et al. in 2004 [63] provided a systematic review of 15 RCTs (n = 1025 patients) evaluating the use of various opioids (including morphine 30–120 mg, methadone 15 mg, and oxycodone 20–45 mg) vs. placebo in the treatment of both musculoskeletal and neuropathic CNCP states. Greater than 30% of reported improvement in pain was reported in the treatment groups regardless of etiology. The authors concluded that there was insufficient evidence to draw any conclusions regarding long-term efficacy due to the brevity of the trials involved (the majority involved no more than 8 weeks of study).
Devulder et al. in 2005 [64] reviewed 11 studies, comprising six RCTs and five observational studies, and reported moderate-quality evidence suggesting improvements in functional outcomes and quality of life in patients with CNCP treated with intermediate (12 weeks) to long-term (4 years) opioid therapy. Pain outcomes were not addressed, and the authors admit to the absence of solid, objective measured variables. Further, more rigorous investigations were recommended.
Furlan et al. in 2006 [65] published a meta-analysis of over 6000 patients in 41 RCTs with opioid treatment lasting up to 4 months. This interesting analysis showed an improvement in pain in patients treated with strong opioids (morphine and oxycodone) but no improvement in function. Conversely, patients treated with weak opioids (tramadol, codeine, and propoxyphene) or non-opioids (naproxen and nortriptyline) reported no improvement in pain but did show improvements in functional outcomes.
Martell et al. in 2007 [66] reviewed 15 studies (n = 1008 patients) including ten RCTs all examining the question of whether opioids (including tramadol, codeine, dextropropoxyphene, morphine, oxycodone, oxymorphone, or fentanyl) improved chronic low back pain. Data from four of the RCTs were pooled into a meta-analysis showing no statistically significant improvement in low back pain with opioid use; furthermore, a high prevalence of substance use disorder (up to 45%) was noted among the populations studied with up to 24% showing aberrant use of prescribed medications.
Trescot et al. provided guidelines from the American Society of Interventional Pain Physicians (ASIPP) in 2008 [67] including a broad analysis looking at the effectiveness of COT as a class but also evaluated evidence for specific agents. They concluded that weak evidence exists for some improvement in both pain and functional outcomes with the use of both morphine and fentanyl, but such evidence is lacking for other opioids, including the most commonly prescribed agents hydrocodone and oxycodone (and combination acetaminophen products).
Noble et al. in 2010 [68], in a Cochrane Review, examined 26 studies on COT in CNCP (only one which was an RCT) with nearly 5000 patients total. Meta-analysis technique was used when appropriate; they report that weak evidence suggested that patients who were able to tolerate long-term opioid therapy in the absence of limiting adverse effects (which group comprised the minority) experience clinically significant but unquantified pain relief, without any evidence of improvement in quality of life or functional outcomes.
Papaleontiou et al. (2010) performed a systematic review and meta-analysis of 31 RCTs and 12 observational studies comparing opioids to placebo or non-opioid analgesics in CNCP, with 10,545 patients in total [69]. Inclusion criteria included an age equal to or greater than 60 years old (mean age 64). The majority of studies were of 4 weeks’ duration or less; roughly two-thirds of the studies evaluated low-potency opioids such as tramadol and codeine. While cautioning that the majority of studies were sponsored by the pharmaceutical industry, the authors report that in older adults with chronic pain and no significant comorbidity, short-term use of opioids is associated with reduction in pain intensity and better physical functioning but poorer mental health functioning. They also concluded that long-term safety, efficacy, and abuse potential of opioids in older adults require further study.
Whittle et al. in 2011 [70] in another Cochrane Review assessed 11 controlled trials (n = 672 patients) comparing opioid therapy to placebo or other non-opioid analgesics specifically for rheumatoid arthritis. They report a weak but statistically significant improvement in analgesia (with NNT of 6) from opioids compared to placebo, but none was seen comparing opioids to NSAIDs. They also concluded that there is lack of evidence of any benefit of opioid use beyond 6 weeks.
Furlan et al. in 2011 updated their earlier analysis with 21 additional studies for a total of 62 RCTs comparing opioids vs. placebo or other drugs for CNCP in 11,927 patients [71]. The purpose of this subsequent investigation was primarily to evaluate whether enriched enrollment randomized withdrawal trials (EERW, a type of RCT in which potential participants receive the study drug on a trial basis prior to randomization into the actual study) provided improved data quality by augmenting the pool with participants with improved tolerance; subanalyses focused on adverse effects and are discussed in Chap. 3. As far as efficacy, the authors report small effect size of positive benefit of opioids on function and medium effect size of positive benefit of opioids on pain; however, they note that the majority of studies lasted 6 weeks or less, were sponsored by the pharmaceutical industry , and contained a large number of dropout subjects. As such they advise that no conclusions can be drawn regarding long-term use.
In 2012 Manchikanti et al. updated the earlier ASIPP position paper from Trescot et al. referenced above and provided an extensive two-part reference, the first part of which [72] presented data on numerous topics including rates of nonmedical use/substance abuse, opioid prescribing patterns, adverse effect review, short- and long-term efficacy review, review of individual agents, and review of specific populations. There were no substantive changes between the conclusions drawn in 2008, with the society reporting fair evidence of pain relief and quality of life benefit for opioid therapy in the short term but limited evidence for benefit in long-term situations (>3 months) and fair evidence that chronic use or long-acting agents confer frequent complications.
Chaparro et al. in 2013 [73] in another Cochrane Review evaluated the role of opioids in chronic low back pain. Fifteen RCTs (n = 5540 patients) comparing opioids to placebo or non-opioid analgesics or antidepressants were included. Strong opioids (tapentadol, morphine, oxycodone, oxymorphone, hydromorphone) were shown to be more effective than placebo in lowering pain reports and improving function (with the caveat that the studies reviewed showed “limited interpretability of functional improvement). Compared to non-opioid analgesics and antidepressants, however, there were no statistically significant differences between the classes in terms of analgesia or functional improvement. The authors concluded that there are no good-quality data (placebo-controlled RCTs) supporting either safety or efficacy of COT in chronic low back pain.
Sehgal et al. in 2013 [74] synthesized data from 144 articles (including 14 RCTs exceeding 3 months’ duration, 9 uncontrolled trials exceeding 3 months’ duration, and 13 systematic reviews) and categorized their analysis by evidence for pain relief, functional improvement, and adverse effects. Their conclusions include:
Among chronic pain patients who respond favorably to opioid therapy (which comprise fewer than half of the patients studied), an average of 30% decreased pain scores was reported.
There is no evidence for long-term efficacy of COT, and conversely there is strong evidence of a high rate of adverse effects including death.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








