Chapter 34
Arrhythmias (Tachycardias) 
General Approach to Tachyarrhythmias in the ICU Setting
Overview
Recommendations for acute treatment can come only after a rapid but accurate assessment of the arrhythmia, its consequences, and its potential causes (Box 34.1). Whereas the hemodynamic consequences of the tachycardia dictate the urgency of treatment, timely assessment of multiple other factors is also essential.
Diagnostic Tools
The appropriate intervention for a tachyarrhythmia depends on obtaining adequate information to arrive at the correct ECG diagnosis (Table 34.1). A reference or baseline 12-lead ECG during sinus rhythm should be sought immediately. This may provide important information regarding underlying heart disease, conduction abnormalities, and baseline P wave morphology. A 12-lead ECG during the tachycardia provides further information regarding the AV relationship during tachycardia, morphologic features of the QRS complex, and P wave morphology. Long telemetry rhythm strips are also helpful in determining the AV relationship, regularity of the ventricular response, and initiation and termination of the tachycardia (Table 34.2). Additional information regarding the AV relationship may be obtained by recording directly from epicardial pacing leads in the postoperative cardiac patient or from permanent endocardial pacing leads.
Table 34.1
Differential Diagnosis of Sustained Supraventricular Tachycardia∗
| Rhythm (Figure Number) | Key Features | Response to Adenosine or Vagal Maneuver |
| Sinus tachycardia (34.2A) | Upright P wave in II, III, aVF; inverted P wave in aVR | No effect or transient slowing |
| Atrial fibrillation (34.2B) | No repetitive organized atrial activity; irregularly irregular ventricular response | Transient slowing of ventricular response |
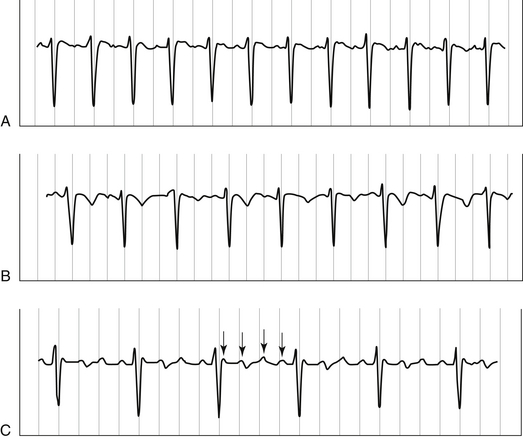
| Atrial flutter (34.2C) | P wave activity 260–300 beats per minute; ventricular response 2:1, but higher-grade AV block possible | Transient slowing of ventricular response with unmasking of flutter waves (see Figure 34.1) |
| Multifocal atrial tachycardia (34.2D) | Multiple (≥ 3) discrete P wave morphologies with isoelectric interval between P waves | No effect or transient AV block |
| Atrial tachycardia (34.2E) | P wave morphology distinct from sinus P wave; may have variable AV block | Transient AV block; termination of tachycardia is possible |
| Atrial tachycardia of digoxin toxicity | Upright P wave in II, III, aVF, V1; typically with variable AV block and ventriculophasic effect | Transient AV block |
| Junctional tachycardia of digoxin toxicity | Regular RR interval; no association between P wave and QRS complex | No effect |
| AV nodal reentrant tachycardia (34.2F) | P wave may only be visible at the end of the QRS complex (pseudo R wave in V1; pseudo S wave in II, III, aVF) | Termination of tachycardia |
| AV reentrant tachycardia (34.2G) | P wave is seen after the QRS complex; inverted P wave in II, III, aVF | Termination of tachycardia after the P wave |
∗Ranked in order of frequency of occurrence in ICU patients.
TABLE 34.2
Electrocardiographic Clues from Initiation and Termination of Supraventricular Tachycardias
| Rhythm | Initiation Phase | Termination Phase |
| Sinus tachycardia | “Warm-up” in atrial rate | Atrial rate slows gradually without an abrupt stop |
| Atrial flutter | Transient AV block may reveal flutter waves (see Figure 34.1) | |
| Atrial tachycardia | Acute change in rate, followed by warm-up in atrial rate with changing PR interval; transient degree of AV block during which P waves appear | Oscillations in PP interval precede changes in RR interval; terminates after QRS complex |
| Junctional tachycardia | No APC triggering the tachycardia | |
| AV nodal reentrant tachycardia | APC followed by a significantly prolonged PR interval and then supraventricular tachycardia; BBB only transient following initiation | Terminates after P wave with adenosine |
| AV reentrant tachycardia | APC and slightly prolonged PR interval; late coupled VPC; persistent BBB; fixed RP relationship | Oscillations in RR interval precede changes in PP interval; terminates after P wave with adenosine; may terminate after a single, especially late coupled, VPC |
Interventions during tachycardia can also provide useful information. Slowing AV nodal conduction with vagal maneuvers or pharmacologic agents can reveal previously masked flutter waves or the P waves of atrial tachycardia (Figure 34.1). Adenosine, an endogenous nucleoside, interacts with specific receptors on the extracellular membrane acutely slowing AV conduction. Because adenosine has a half-life of only 0.5 to 5 seconds, doses (6 mg and then 12 mg) must be given in a rapid IV bolus injection followed by a flush. Even administration through a peripheral intravenous line can attenuate its effects. The short-lived adverse effects of adenosine include facial flushing, chest pain or pressure, bronchospasm, and dyspnea. By briefly but effectively blocking the AV node, adenosine is capable of terminating AVNRTs. Adenosine is not a perfect diagnostic tool, however, because it may also terminate some episodes of focal triggered atrial tachycardia and VT.
The “When in Doubt, Knock It Out” Rule
Severe hemodynamic instability resulting from a tachycardia warrants prompt electrical cardioversion. Before cardioversion, however, one needs to consider that the tachycardia may be sinus tachycardia or multifocal atrial tachycardia, neither of which responds to electrical cardioversion. In addition, the hemodynamic instability may be due to a separate cause, such as blood loss or sepsis, and the tachyarrhythmia is just a secondary event. Once the decision is made to perform electrical cardioversion, patient comfort should be ensured by administering a short-acting IV benzodiazepine, such as midazolam, or the anesthetic agent propofol (see Chapter 5).
One must choose a mode of delivery (synchronous or asynchronous) and an energy level. Delivery of shock energy for ventricular fibrillation (VF) and polymorphic VT should be performed in the asynchronous mode. For all other arrhythmias, energy should be delivered in the synchronous mode to decrease the risk of causing arrhythmia degeneration or VF. For any rhythm with dire hemodynamic consequences, energy output should begin at 200 joules and then should be increased to 300 joules or the maximum available dose from the defibrillator if the initial attempts fail (see Appendix D for Advanced Cardiac Life Support [ACLS] algorithms). Only in the stable patient should lower energy levels be attempted.
Narrow Complex Tachycardias
Supraventricular tachycardia (SVT) is a commonly used but imprecise term. By definition, all narrow complex tachycardias, including sinus tachycardia, are supraventricular in origin because only depolarization over the His-Purkinje system results in a narrow QRS. The frequency of the different SVTs occurring in the ICU setting differs from that seen in the emergency department or outpatient office (Figure 34.2 and see Table 34.1). Underlying diseases, heightened sympathetic tone, and use of medications, such as inotropes, theophylline, or digoxin, can precipitate these tachyarrhythmias. Clues to their ECG diagnosis can be found in rhythm strip analysis with recording of the onset and termination of tachycardia (see Table 34.2). More detailed recommendations of pharmacologic and nonpharmacologic management strategies for specific SVTs are discussed next.
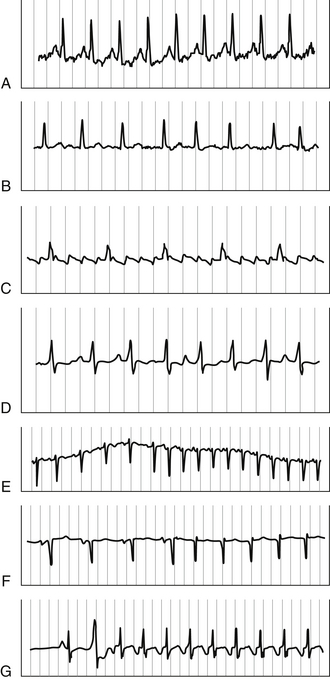
Figure 34.2 A, Sinus tachycardia. B, Atrial fibrillation. C, Atrial flutter. D, Multifocal atrial tachycardia. E, Atrial tachycardia. F, Atrioventricular nodal reentrant tachycardia (AVNRT). G, Atrioventricular reentrant tachycardia (AVRT). See Tables 31.2, 34.1, 34.2, and 31.3 and the text for diagnostic features of each tachycardia.
Sinus Tachycardia
The most common SVT encountered in the ICU is sinus tachycardia, which occurs in response to underlying conditions such as pain, fever, infection, anemia, pulmonary embolism, thyrotoxicosis, myocardial ischemia, and congestive heart failure (CHF) (see Figure 34.2A). Autonomic dysfunction may also play a role, particularly in certain neurologic disorders, such as Guillain-Barré syndrome. The clinical challenge lies in making the diagnosis of sinus tachycardia and recognizing its significance as a secondary problem. It should be viewed as a warning sign of underlying abnormalities and prompt a thorough evaluation. Treatment is directed toward the underlying cause.
Atrial Fibrillation and Atrial Flutter
Atrial fibrillation and atrial flutter are similar arrhythmias that occur commonly in the ICU (see Figure 34.2B and C). Risk factors for the development of atrial fibrillation include advanced age, CHF and valvular heart disease, systemic hypertension, pulmonary disease, sleep apnea, and thyrotoxicosis. Acute respiratory failure and high sympathetic tone, particularly in patients with other risk factors, can precipitate a paroxysm of atrial fibrillation or flutter. On the 12-lead ECG, atrial fibrillation is marked by the replacement of organized atrial activity with an irregular undulating baseline of variable amplitude. At times, the undulations may be coarse and mimic atrial activity; however, these coarse waves are not truly cyclical: the RR interval is always irregular and characteristically some will occur < 200 msec apart.
In contrast, atrial flutter is due to a reentrant mechanism within the atria and results in regular flutter waves (typically at a rate of 240 to 300 beats per minute [bpm]) on the 12-lead ECG. In the absence of medications, the ventricular rate is typically regular, with 2:1 conduction of flutter waves, resulting in a typical regular ventricular rate of 130 to 150 bpm. In fact, when encountering a regular, narrow complex tachycardia at the rate of 130 to 150 bpm, one should always first consider the diagnosis of atrial flutter. It is frequently more difficult to control the ventricular rate in atrial flutter than in atrial fibrillation because of the slower and more organized impulses reaching the AV node. Flutter waves can be easily overlooked, and blocking conduction in the AV node can help unmask them (see Figure 34.1). If atrial wires/leads (temporary or permanent) are present, direct recordings from these wires can help clarify the diagnosis.
A number of pharmacologic agents are available to control the ventricular response during atrial fibrillation and flutter (Table 34.3). Once the rate is controlled and the patient is hemodynamically stable, cardioversion to sinus rhythm can be pursued on an elective basis. The timing of elective cardioversion is largely determined by the need to reduce the risk of thromboembolic complications. The lack of organized atrial activity associated with atrial fibrillation leads to circulatory stasis within the atria and can result in formation of atrial thrombi and embolism into the systemic circulation. Even in new-onset atrial fibrillation, there is some embolic risk. If a patient has no contraindications, anticoagulation should be started as soon as possible, certainly within 24 to 48 hours if spontaneous conversion to sinus rhythm has not occurred. If a patient cannot receive anticoagulation, prompt cardioversion within 24 to 48 hours should be considered. In addition, patients with known risk factors for stroke who are in atrial fibrillation for over 24 hours may require a transesophageal echocardiogram to exclude the presence of a thrombus in the left atrial appendage before cardioversion.
TABLE 34.3
Treatment of Atrial Fibrillation and Flutter: Ventricular Rate Control

∗For specific doses of digoxin based on renal function, see Table 17.2, Chapter 17.
Atrial fibrillation and flutter respond to the same antiarrhythmic drugs outlined in Table 34.4. In fact, atrial fibrillation frequently organizes into stable atrial flutter on these medications. It is common, however, for these arrhythmias to resist pharmacologic interventions and require electrical cardioversion. Despite this, the use of antiarrhythmic agents before electrical cardioversion is indicated because drug treatment may help maintain sinus rhythm after cardioversion. In patients whose condition is stable, electrical cardioversion of atrial fibrillation typically begins at 200 joules, but higher doses of energy may be required. It is always performed in the QRS synchronous mode. Cardioversion of atrial flutter generally requires significantly lower energies, usually 50 to 100 joules, and again should always be delivered in the synchronous mode. An amnestic agent, such as midazolam, should always be given beforehand.
TABLE 34.4
Treatment of Atrial Fibrillation and Flutter: Conversion to Sinus Rhythm
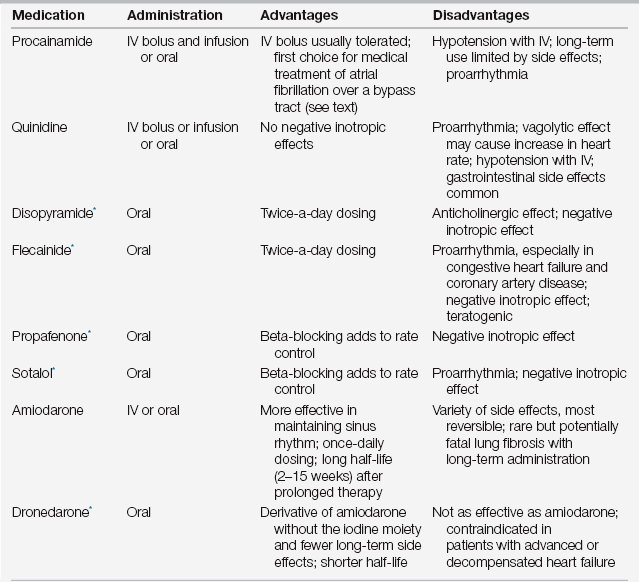
∗Consider cardiology consultation before using these agents.
Some patients have a clear precipitating factor for atrial fibrillation, such as pulmonary embolism or pneumonia, and do not require long-term medical management. Spontaneous conversion to sinus rhythm may occur as the patient recovers from the acute insult. Other patients who have underlying risk factors remain at risk for recurrence and may require chronic antiarrhythmic management and anticoagulation. The patient with atrial fibrillation and the WPW syndrome is discussed later.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree