Fig. 31.1
Representative of cortical SSEP changes caused by unilateral carotid occlusion due to retractor malposition
What are the possible causes for the diminished left-sided SSEP and MEP signals at this point in time?
Given that this change is focal (not global) in nature, anesthetic and physiologic causes for the diminished signals are less likely. Hence, surgical, technical, or positional causes should be sought to explain this evoked potential change. In this case, the anesthesiologist raised the blood pressure by 20 % above its current level while troubleshooting other causes. The position of the arms was checked while the neuromonitoring technologist was assessing the technical fidelity of the signals. There were no apparent technical or positional problems, leaving us only with a potential surgical cause for these changes. The surgical causes for signal changes may be related to mechanical stress, thermal injury, surgical injury, or ischemia. Mechanical stresses are usually associated with EMG discharges and are related to either nerve root irritations or dural insults. Neither instrumentation nor thermal devices were being used on any of the neural structures at this time. Ischemia to the left arm could explain MEP changes but it would not explain the SSEP changes in the lower extremity nor those in the upper extremity (since the response from Erb’s point was normal). In fact, occlusion of the right-sided cerebral blood supply is a more likely cause of the changes that were seen. An ischemic or hemorrhagic stroke to the right hemisphere, caused by manipulation of an atherosclerotic right carotid artery, is a possibility. Even more likely is cerebral ischemia caused by obstruction of the right carotid artery, which is in close proximity to the surgical field and may be distracted by the surgical retractor.
Absent blood flow within the right carotid artery was confirmed by the anesthesiologist by palpation of the right superficial temporal artery (transcranial Doppler could also have been used for this indication). The surgeon was informed and repositioned the retractors, which resulted in an immediate restoration of the right superficial temporal pulse as well as both the MEP and SSEP waveforms.
Case 2
A 36-year-old, ASA PS 1, woman without significant past medical history is scheduled for a C5–C7 ACDF for disk herniation and removal of an osteophyte. Anesthesia is performed with standard ASA monitoring and neurophysiologic monitoring consisting of EMG recorded from the deltoid, biceps, and triceps, SSEPs, and MEPs for the upper and lower extremities. Normal baselines of all monitoring parameters were obtained prior to surgery. Discectomies at the C4–5, C5–6, and C6–7 interspaces were performed and significant bony overgrowth along the pedicles of the vertebral canal was removed. At one point, during shaving along the pedicle of C5, there was a burst of spontaneous EMG activity recorded from the biceps (Fig. 31.2).
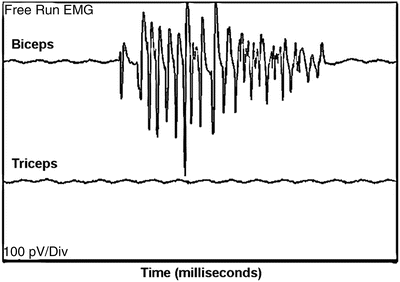

Fig. 31.2
Spontaneous EMG firing at the biceps, with minimal noise in other muscles
What could be the cause of this EMG change ?
EMGs are used during such operations to continuously monitor for mechanical irritations to the spinal cord or nerve roots induced by the different surgical instrumentation used. EMG discharges are related to mechanical insults that cause depolarization, and are not related to ischemia. The premise of using EMG monitoring is to alert the surgeon to changes related to such mechanical irritation before a greater insult occurs that can lead to more permanent neuronal damage. Notably, “light” anesthesia can be a cause for abnormal EMG discharges that are not surgically related (however, light anesthesia usually produces activity in multiple muscles rather than the one muscle noticed here). The use of other electrical devices, such as cautery, may also produce “false” EMG discharges. EMG discharges have been graded in intensity according to a four-level system [16]. In this case, the discharges were mild, the surgeon was notified, and the discharges disappeared immediately thereafter. The surgeon continued to work but a few minutes later severe discharges reappeared (which might indicate the potential for a larger mechanical and/or ischemic insult). The surgeon was again notified and paused surgical activity while MEPs were obtained. During this time, SSEPs were also being acquired. These modalities were used as confirmatory tests in the presence of the EMG changes, testing for any potential spinal cord injury that might be caused by ischemia as a result of mechanical distortion.
MEPs were acquired and revealed no changes. SSEP responses were also stable. How should we proceed?
Because the only change seen in the neurophysiologic monitoring was an increase in EMG activity at C5 or C6, there is most likely a surgical reason for the observed change. In this case, the most likely scenario is that the C5 or C6 nerve root emerging from the intervertebral foramen has been mechanically irritated during attempted decompression at the foramen. EMG provides a real-time alert for impending neurologic deficits related to a mechanical insult. The SSEP and MEP waveforms were most probably not affected because they tend to transmit along major peripheral nerves, which originate from many individual nerve roots, thus masking irritation or impending injury to a single nerve root.
A disadvantage of EMG monitoring compared to MEP monitoring is that it can be “contaminated” by artifact from various sources, including patient movement, Bovie interference, etc., while this is not the case with the relatively high amount of stimulation needed to generate MEPs. A potential advantage of EMG monitoring compared with MEP monitoring, as was seen in this case, is the continuous nature of EMG signal acquisition, which might detect compression/injury to a nerve with greater sensitivity than an evoked MEP, whose acquisition is intermittent and would require deliberate acquisition at or after the time of the insult to the nerve to detect it. Most importantly, as illustrated in this case, EMG also has the advantage of being able to detect irritation to a single nerve root, which is less likely with either SSEP or MEP monitoring, because these modalities monitor major mixed sensory/motor nerves and muscles that have overlapping nerve root innervation.
The surgeon stopped working in the C5–C6 nerve root area and the EMG recording returned to a silent state. The surgeon proceeded to complete the surgery without any further changes in the neuromonitoring signals. The patient was awakened, extubated, and examined neurologically, with no change in the examination as compared with her preoperative status.
Case 3
A 47-year-old woman, 140 kg, ASA PS 2 with a past medical history of morbid obesity, is scheduled for a C3–C5 ACDF (right-sided approach) for intermittent and nonreproducible radiculopathic symptoms in her left upper arm. Her clinical examination is not consistent with any myelopathy, and a cervical MRI seems to confirm this (no spinal cord impingement). Of note, on her physical examination, the patient has a Mallampati Class IV airway with a thyromental distance of 4 cm. Previous anesthetic records indicate that she was easy to ventilate by bag/mask but difficult to intubate, requiring fiberoptic intubation.
How should the airway be secured in this patient? Should an awake or asleep technique be used? Would neuromonitoring, after induction but prior to intubation, be of any value in this case? What neuromonitoring modalities should be used for this case?
Based on the patient’s previous airway history, anawake or asleep fiberoptic technique would seem prudent. The advantage of an awake fiberoptic intubation, besides maintaining spontaneous ventilation, would be to retain the ability to examine the patient for evidence of new radiculopathic/myelopathic symptoms during and after intubation. An asleep fiberoptic intubation could also be performed, with SSEPs and EMG acquired pre- and postintubation (under “steady state” anesthesia), to confirm that neurologic injury from intubation had not occurred. Whether intubation is performed awake or asleep, the use of flexible fiberoptic bronchoscopy should limit the amount of neck movement and cervical subluxation compared to a direct laryngoscopy.
For this surgical procedure, any combination of the neuromonitoring modalities mentioned above could be used depending on the level of concern for spinal cord, nerve root, orperipheral nerve injury .
Because no myelopathy is suspected in this patient, and because of a known ability to mask ventilate her in the past, an oral asleep fiberoptic intubation is chosen to secure the airway.SSEPs of the median and posterior tibial nerves are obtained as well as EMG of the deltoid, biceps, and triceps muscles. All of these neuromonitoring modalities remained unchanged before and after intubation under a “steady state” of anesthesia, being careful to record signals after recovery from the succinylcholine (i.e., no residual effect on EMG) used to facilitate intubation. A small amount of rocuronium (20 mg) was then given to assist during positioning and exposure. Anesthesia was maintained with propofol, 100–150 μg/kg/min, and fentanyl, 1–5 μg/kg/h (TIVA), which were infused through a dedicated intravenous (IV) catheter placed in the left arm. Fluids and bolus medications were injected into the IV catheter placed in the right arm. SSEP baselines were obtained from all four extremities and were found to be robust and reproducible. Before surgical incision, the neuromonitoring technologist reports a greater than 50 % decrease in the amplitude and an increase in the latency of the SSEP signals recorded from the right arm (Fig. 31.3).
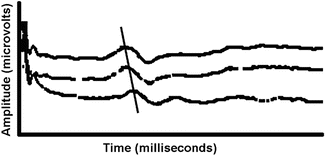

Fig. 31.3
SSEP changes at the cervical level due to excessive traction on the shoulder caused by taping too tightly
What could be the cause of these right arm SSEP changes ? What should be done to correct these changes and avoid injury?
Since surgery has not yet begun, surgical causes for the observed changes are eliminated, and because this is a unilateral change, anesthetic and physiologic causes are unlikely. The possibility of a technical cause exists (e.g., due to a decrease in stimulation intensity caused by partially dislodged stimulating pads). All stimulating and recording pad placements were checked, and all other technical parameters were within normal limits. On further evaluation of the right shoulder position, the shoulder was found to be taped down to the table rather tightly, placing it in undue traction. The tape was somewhat released, which resulted in the return of the SSEP signals to baseline. Presumably, the observed change was related to stretching of the brachial plexus, and if left uncorrected might have led to a longer-lasting neurapraxia. A similar effect could result from straps attached to the wrists to allow traction, which would improve visualization of the spine under fluoroscopy. Other causes of a change in the SSEP responses from the upper arm were also excluded (such as a tourniquet effect of the noninvasive blood pressure cuff or drapes used to hold the arm or a cold arm from infusing cold intravenous fluids).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








