Introduction
In recent years, the development and application of new animal models of disease processes has been a popular scientific trend [1]. However, few of these models have focused on sexuality, and fewer still have modeled pain conditions that impact sexuality. Urogenital and abdominal pain conditions associated with dyspareunia impact a staggering percentage of women, yet very few of these conditions are well understood. Although imaging studies have greatly advanced human research in this area [2], experimental options using human subjects are still limited. Animal models allow experimental manipulations to evaluate the causal relationships between pathological causes and physiological effects. These models are convenient and cost-effective, and they permit the testing of hypotheses that are otherwise ethically implausible in humans. The development of viable animal models for conditions that are associated with painful intercourse, such as en-dometriosis, interstitial cystitis (IC), irritable bowel syndrome (IBS), and provoked vestibulodynia (PVD) might have profound implications for our understanding of the etiology, maintenance, and treatment of these debilitating conditions.
Evaluation of Animal Models of Pain
Pain in animals is defined as “an aversive sensory experience caused by actual or potential injury that elicits progressive motor and vegetative reactions, results in learned avoidance behavior, and may modify species-specific behavior, including social behavior” [3]. Animals cannot verbally rate their pain intensity, quality, or location, nor can they communicate the impact of emotion on pain. Instead, researchers infer the presence of pain from abnormal behaviors that are (hopefully) unique to the experimentally induced nociceptive state. The difficulty in measuring an animal’s emotional or cognitive responses to pain suggests that we are largely using nociceptive models, rather than true pain models [4]. However, just because we can’t measure something doesn’t mean it isn’t there. Nevertheless, the word pain will be used throughout this chapter.
Pain can be typified as spontaneous or provoked, depending on whether or not it is elicited by exogenous stimulation. Many existing animal models of pain are limited in their duration; chronic, spontaneous pain–the most clinically relevant form–has proved particularly difficult to model in animals [5]. Pain conditions can also be visceral or somatic in nature. Visceral pain originates from the internal organs contained within the chest and abdomen, and it is characterized by increased autonomic reactivity, emotional salience, and diffuse pain that may be referred to other visceral or somatic tissue that shares common innervation at the level of the spinal cord [6]. Referred pain is perceived in areas distal from the site of injury that receives common spinal input as the region where pain originates [7, 8]. The majority of animal models of pain conditions associated with dyspareunia are visceral in nature, including uterine inflammation, vaginal and uterine distension, endometriosis, and abdominal pain (including cystitis and colitis).
In animal models, behavioral responses may reflect the location of the pain in the case of somatic tissue (e.g., withdrawal of a heated hind paw), whereas visceral pain may be manifested as referred somatic pain [7]. Patterns of pain behavior can increase in frequency or magnitude with higher levels of noxious stimulation. Abnormal behaviors that show temporal correspondence with tissue injury or inflammation are thought to reflect injury-specific pain, although the correlation may not be strong. For visceral pain in particular, the absence of behavior may be indicative of pain, as evidenced by reduced mobility or motivation to engage in normal activity [9]. In addition, estrous cyclicity may significantly impact some behaviors [10], but many indices of behavioral pain show equal variability when male versus female animals are used [11]. Empirical validation that a behavior is specific to pain is often achieved via the administration of known analgesics, such as nonsteroidal anti-inflammatory drugs, lidocaine, or morphine.
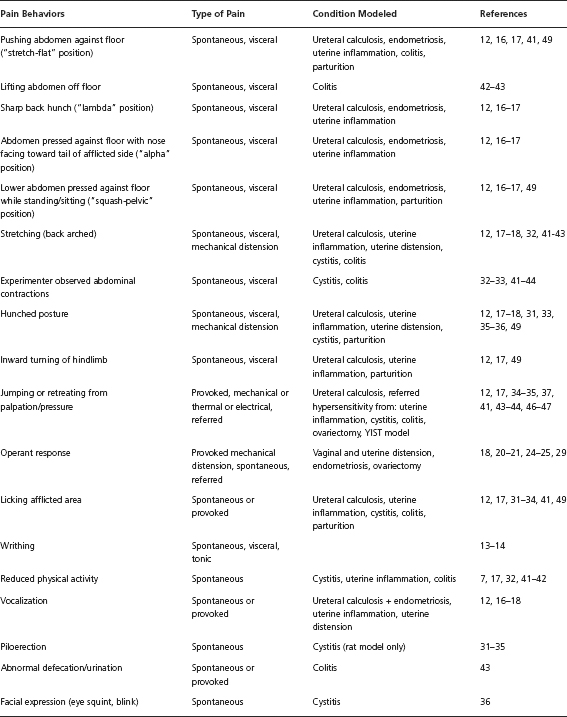
Ultimately, many behaviors have been associated with pain in rodents. Table 30.1 lists behaviors that have been linked to animal models of dyspareunia. Notably, the criteria for dyspareunia vary between models. Whereas some models directly measure vaginal sensitivity to noxious stimuli, other models induce pain conditions associated with dyspareunia. Ideally, pain behaviors are unique to an experimental manipulation, easily quantifiable with minimal need for interpretation, frequent enough to allow for statistical comparisons between groups, and reliably observed in afflicted animals (and rare in healthy animals). Such behavior should coincide with the duration and severity of injury and be mitigated by analgesics in a dose-dependent manner. Most importantly, the validity of an animal model of pain relies on whether the researchers have accurately identified a pain behavior that closely parallels the clinical characteristics of the condition it is intended to model. This chapter will be limited to reviewing rodent models of female urogenital and abdominal pain that include the measurement of pain behavior, not electrophysiological or electromyographic proxies, as a primary outcome measure [4].
Ureteral Calculosis
Women with dysmenorrhea, or painful menstruation, often report dyspareunia and are more likely to experience urinary calculosis (kidney stones). Based on this comorbidity, animal models of ureteral calculosis (UC) may in-directly induce dyspareunia, although this link has never been formally tested.
The first detailed behavioral characterization of UC-induced visceral pain was conducted by Giamberardino’s laboratory [12]. Within a day of implantation of an artificial stone into the left ureter, rats displayed a variety of spontaneous pain behaviors including stretching, hunched back, abdominal/flank licking, flank muscle contractions accompanied by ipsilateral inward hindlimb motions, lower abdominal squashing (against the floor), and the adoption of a supine position with the left hindlimb retracted into the abdomen. These behaviors slowly decreased in frequency and duration over four days postimplantation. Rats with frequent visceral pain behaviors were more likely to vocalize to electrical stimulation of the ipsilateral oblique muscles, indicative of referred pain. These behaviors are similar to the protracted abdominal stretching observed in early visceral pain models [13, 14]. Pain behaviors were reduced with intraperitoneal 5 mg/kg/day morphine. Giamberardino’s model established a typology for abnormal pain behaviors associated with visceral pain that would be replicated or modified by the majority of subsequent abdominal visceral pain models.
Based on preliminary human evidence linking dysmenorrhea, endometriosis, and UC [15], Giamberardino and colleagues [16] developed a dual rat model of endometriosis with UC to investigate whether abdominal pain behaviors found in either condition are enhanced by the comorbidity. Animals with endometrial autografts plus stone implantations showed significantly longer bouts of pain behavior compared to stone implantation only or sham groups. Although all animals developed some referred hyperalgesia caused by the presence of a ureteral stone, the endometriosis + UC group displayed the greatest magnitude of referred pain as indicated by reduced vocalization threshold in response to electrical stimulation of the left oblique muscles.
Uterine Inflammation
Wesselmann and colleagues [17] characterized pain behavior associated with uterine inflammation in the rat. The pain behaviors they examined were based on the Giamberardino model of UC [12]. To induce inflammation, 10% mustard oil and a mineral oil vehicle were injected into the uterine lumen, and pain behaviors were videotaped for seven days postsurgery. Of animals receiving uterine inflammation, 79% displayed prolonged periods of spontaneous pain behavior, with behavior frequency peaking two days after surgery. Dramatic individual differences were found in the frequency and duration of pain behaviors, and animals with uterine inflammation showed reductions in overall mobility. Of animals displaying spontaneous pain behavior, 66% also showed referred muscle hypersensitivity in the lower back and flanks that actually outlasted the occurrence of spontaneous pain behaviors.
Wesselmann’s study was especially significant in that it established that pain from distinct viscera–the ureter and the uterus–resulted in very similar behaviors, including behavioral evidence of referred pain. Although this behavioral similarity may support the validity of these behaviors as being specific to pain, it also indicates that the behaviors are not specific enough to distinguish between visceral pains of different origins. The poor localization of visceral pain, however, makes it very unlikely that different visceral pains would be manifested in unique behavioral patterns.
Vaginal and Uterine Distension
Berkley and colleagues [18] established one of the earliest rat models of reproductive tract pain using vaginal and uterine distension. The elegance of this model relies on the novel operant task devised by the authors, which required rats to learn that a discrete behavioral response (extending the nose to interrupt a photocell circuit) would terminate an aversive stimulus (vaginal or uterine mechanical distension with a latex balloon). The authors argued that the rats’ motivation to perform the escape behavior in response to high levels of distension indicated that intense mechanical distension constituted an aversive stimulus to the rats. The intense level of stimulation employed by this model is in contrast to innocuous levels of vaginal stimulation, which have positively reinforcing and analgesic properties in rodents [19].
Berkley et al. [18] validated this behavioral pain model in adult virgin female rats with low levels of ovarian hormones (i.e., Metestrus), to control for the potentially confounding effects of estrous cycle hormone fluctuations. Rats reliably escaped distension with increasing speed and frequency as the vaginal distension volume increased, and this response pattern held throughout the estrous cycle [20]. The rats’ ability to detect and escape from uterine distension, however, was less predictable–many rats produced operant responses during control trials when distension volumes were minimal, and a large minority of animals did not show behavioral discomfort with maximum levels of uterine distension. The authors noted that rats often responded to uterine distension with stretching behavior.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree