KEY POINTS
1. Nonischemic cardiomyopathy (53%) is the most common pretransplant diagnosis worldwide.
2. Nearly 70% of recipients in the United States require some form of life support prior to cardiac transplantation. For example, in the United States, the number of pretransplant patients with ventricular assist devices has risen dramatically over the recent years.
3. Increased preoperative pulmonary vascular resistance in the recipient is predictive of early graft dysfunction and increased mortality related to an increased incidence of right heart dysfunction.
4. Because of intact spinal reflexes, transplant donors may exhibit hypertension, tachycardia, and muscles movement. These responses do not indicate cerebral function or pain perception.
5. During orthotopic cardiac transplantation, the cardiac autonomic plexus is transected, leaving the transplanted heart without autonomic innervation. Inotropes are utilized in the postbypass period.
6. Right ventricular failure is a significant cause of early morbidity and mortality accounting for nearly 20% of early deaths after transplantation. RV failure and increased pulmonary vascular resistance in the postbypass period can be often challenging for the cardiac anesthesiologist to manage.
7. Cardiac allograft vasculopathy (CAV) is a form of graft failure. Unlike atherosclerotic CAD, CAV is characterized by diffuse intimal hyperplasia.
8. In contrast to the non-transplanted patient, where increases in cardiac output can be quickly achieved through a sympathetically mediated increase in heart rate, the cardiac transplanted patient, whose sympathetic innervation to the heart will be interrupted, tends to require an increase in preload in order to increase cardiac output.
9. Autonomic denervation of the heart alters the pharmacodynamic response of many drugs (Table 16.8). Drugs that act directly on the heart are effective.
10. Regional or general anesthesia has been successfully utilized in the postcardiac transplant patient for surgical procedures. For any selected anesthetic technique, maintenance of preload is essential (see keypoint 8 above).

ALTHOUGH “DESTINATION THERAPY” USING MECHANICAL circulatory support (MCS) devices has increasingly become a viable option, cardiac transplantation remains the gold standard for the treatment of heart failure (HF) refractory to medical therapy [1]. Since the first human cardiac transplant by Christiaan Barnard in 1967, over 89,000 cardiac transplants have been performed worldwide [2]. Currently, approximately 3,500 cardiac transplants are performed per annum, with approximately 2,200 occurring in the United States [2]. Despite an increasingly high-risk patient population, survival rates continue to improve due to advances in immunosuppression, surgical technique, perioperative management, and the diagnosis and treatment of allograft rejection [3]. In the United States, cardiac transplantation is limited to member centers of the United Network for Organ Sharing (UNOS). UNOS, in turn, administers the Organ Procurement and Transplantation Network (OPTN) which maintains the only national patient transplant waiting list in the United States.
I. Heart failure
More than 5 million American adults carry a diagnosis of HF, with an incidence of 670,000 per year [4]. The American College of Cardiology (ACC) and the American Heart Association (AHA) define HF as a clinical syndrome that can result from any structural or functional cardiac disorder that impairs the ability of the ventricle to fill with, or eject blood. The majority of patients with HF owe their symptoms to impairment of left ventricular (LV) myocardial function [5]. Because volume overload is not necessarily present, the term “HF” is now preferred to the older term “congestive HF.”
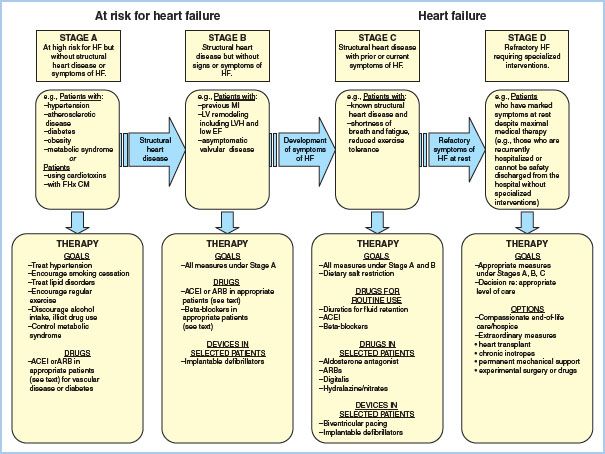
The New York Heart Association (NYHA) scale is used to quantify the degree of functional limitation imposed by HF. Most patients with HF, however, do not show an uninterrupted and inexorable progression along the NYHA scale [5]. In 2005, the ACC/AHA created a staging scheme reflective of the fact that HF has established risk factors, a clear progression, and specific treatments at each stage that can reduce morbidity and mortality (Fig. 16.1). Patients presenting for heart transplantation invariably present in Stage D, refractory HF.
1
A. Etiology
Nonischemic cardiomyopathy (53%) is the most common pretransplant diagnosis worldwide [2]. Ischemic cardiomyopathy accounts for 38%, with valvular cardiomyopathy, retransplantation, and congenital heart disease accounting for the remaining percentage of adult heart transplant recipients.
Figure 16.1 Stages in the development of HF and recommended therapy. (From Jessup M, Abraham WT, Casey DE, et al. Focused update incorporated into the ACC/AHA 2005 guidelines for the diagnosis and management of heart failure in adults. Circulation. 2009;119:e391–e479.)
B. Pathophysiology
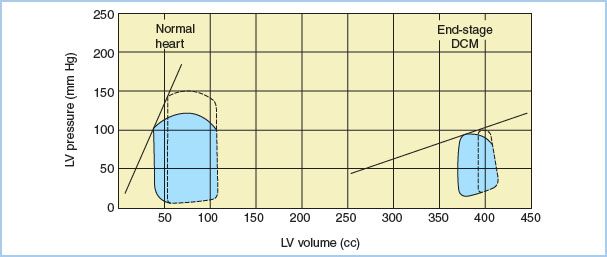
The neurohormonal model portrays HF as a progressive disorder initiated by an index event, which either damages the myocardium directly or disrupts the ability of the myocardium to generate force [6]. HF progression is characterized by declining ventricular function and activation of compensatory adrenergic, and salt and water retention pathways. Ejection fraction (EF) is initially maintained by increases in LV end-diastolic volume, myocardial fiber length, and adrenergically mediated increases in myocardial contractility. LV remodeling takes place during this time, and while initially adaptive, may independently contribute to HF progression [6]. The chronic overexpression of molecular mediators of compensation (e.g., norepinephrine, angiotensin II, endothelin, natriuretic peptides, aldosterone, and tumor necrosis factor) may lead to deleterious effects on cardiac myocytes and their extracellular matrix [6,7]. The result is progressive LV dilation, as well as decreasing EF and cardiac output (CO). Fatigue, dyspnea, and signs of fluid retention develop. Other organ systems such as the liver and kidneys become compromised by persistent decreases in CO and elevated venous pressures. With continued progression of HF, stroke volume (SV) becomes unresponsive to increases in preload, and increases in afterload are poorly tolerated (Fig. 16.2). Chronic exposure to circulating catecholamines may result in downregulation of myocardial b1-adrenergic receptors, making the heart less responsive to inotropic therapy.
Figure 16.2 Pressure–volume (P–V) relationships in a normal heart and a heart with end-stage dilated cardiomyopathy (DCM). Shown are the LV P–V loops (dotted lines) obtained from a normal heart and a heart with end-stage DCM following an increase in afterload. The slope depicts the LV end-systolic P–V relationship. Note that the myopathic heart SV is markedly decreased by increases in afterload. (From Clark NJ, Martin RD. Anesthetic considerations for patients undergoing cardiac transplantation. J Cardiothorac Anesth. 1988;2:519–542, with permission.)
C. Medical management of HF
1. Therapeutic goals
The therapeutic goal for HF management is to slow or halt the progression from Stage A to D. Lifestyle modifications and selected pharmacotherapy are the mainstays of therapy for Stages A and B. When Stage C is reached, combination pharmacotherapy includes diuresis, interruption of the renin–angiotensin axis, and b-blockade. Selective use of direct vasodilators and inotropes is also indicated. Utilization of cardiac resynchronization therapy (CRT) and/or an implantable defibrillator may be recommended. Despite optimum medical management, some patients will progress to Stage D, refractory HF. Chronic intravenous (IV) inotropes, mechanical support devices, and heart transplantation are the only measures available for palliation or treatment.
a. Inotropes
Inotropic agents commonly used to treat cardiac failure include digitalis, catecholamines, and phosphodiesterase-III (PDE) inhibitors. Digitalis, in combination with b-blockers, is effective in treating HF complicated by atrial fibrillation, but does not confer increased survival [5]. Administered orally, digitalis exerts a positive inotropic effect by inhibiting the myocardial cell sodium pump and increasing cytosolic calcium concentrations. Digitalis also prolongs atrioventricular conduction time, leading to a decrease in heart rate. Digitalis blood levels should be monitored as significant side effects including atrial and ventricular arrhythmias can occur, particularly in the presence of hypokalemia.
Myocardial b1-adrenergic receptor stimulation by IV administration of catecholamines, such as epinephrine, norepinephrine, dobutamine, or dopamine, is often used to improve cardiac performance, diuresis, and clinical stability. PDE inhibitors, such as milrinone, may also be used. PDE inhibitors combine both positive inotropic and vasodilatory activity by inhibiting cyclic adenosine monophosphate (cAMP) metabolism. Occasionally, patients may not be weaned from IV inotropic support despite repeated attempts. At such times, an indwelling IV cannula may be placed to allow for the continuous infusion of an inotrope for patients awaiting transplantation, or to facilitate home palliation. However, the use of chronic inotrope has not been shown to increase survival [5].
b. Diuretics
Diuretics provide symptomatic relief to HF patients more quickly than any other class of drug, and are the only class of drug used in HF that can adequately control fluid retention. Classes of diuretics used include the loop diuretics (e.g., furosemide, bumetanide, torsemide) and the thiazide diuretics (e.g., hydrochlorothiazide, metolazone). Adverse diuretic effects include electrolyte disturbances (particularly of potassium and magnesium), hypotension, intravascular volume depletion, and azotemia.
c. Renin–angiotensin–aldosterone system inhibitors
The renin–angiotensin–aldosterone system may be inhibited by angiotensin converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs), or the aldosterone receptor. In combination with b1-antagonists, ACEIs have been shown to reduce HF progression by interfering with the neurohormonal pathways that modulate LV remodeling; ACEIs alleviate symptoms, enhance the overall sense of well-being, and reduce the risk of hospitalization and death in patients with HF [5]. Adverse effects of ACEIs include hypotension, worsening renal function, and hyperkalemia. If the adverse effects of ACEIs cannot be tolerated, ARBs may be considered as an alternative. The propensity of ARBs to increase serum potassium levels limits their usage in patients with impaired renal function. Aldosterone exerts an adverse effect on heart structure and function independent of angiotensin II [5]. Spironolactone is the most widely used aldosterone antagonist in HF patients, although eplerenone has been studied in HF after myocardial infarction.
d. Vasodilators
Vasodilators are used in the acute treatment of HF to reduce myocardial preload and afterload, thereby reducing myocardial work and oxygen demand. Nitrates (e.g., nitroglycerine and sodium nitroprusside) may also be useful for relieving the symptoms of pulmonary edema by reducing ventricular filling pressures and afterload. b-Type natriuretic peptide (nesiritide) is used effectively for the medical management of decompensated HF [5]. Nesiritide, an arterial and venous dilator, acts by increasing cyclic guanosylmonophosphate (cGMP) [8]. In hospitalized patients, the use of nesiritide was associated with a dose-dependent reduction of pulmonary capillary wedge pressure (PCWP), right atrial pressure, and mean pulmonary artery (PA) pressure, along with improvements in cardiac index and clinical outcome [9]. However, a clear benefit in terms of morbidity and mortality has not been demonstrated for the use of nesiritide in acute or chronic HF [5].
e. β-Adrenergic receptor blockade
In combination with interruption of the renin–angiotensin–aldosterone axis and diuresis, b-adrenergic receptor blockade is the standard treatment for HF. Carvedilol, bisoprolol, and sustained release metoprolol have been demonstrated to be effective in reducing mortality in patients with chronic HF [5]. Chronic adrenergic stimulation is initially supportive to the failing heart, but may lead to progression of HF through neurohormonally mediated LV remodeling. b-Blockers likely exert their benefit through attenuation of this influence. Adverse reactions to b-blockers in patients with HF include fluid retention, fatigue, bradycardia, heart block, and hypotension.
f. Anticoagulants
Patients with HF are at increased risk of thromboembolism as a result of low CO and a high incidence of coexistent atrial fibrillation. Long-term prophylactic anticoagulation with agents such as coumadin is common and may contribute to perioperative bleeding at the time of cardiac transplantation.
g. Cardiac implantable electronic devices (CIEDs)
CIEDs broadly consist of devices that seek to manage bradyarrhythmias (pacemakers), tachyarrhythmias (implantable cardiac defibrillators), and ventricular dyssynchrony (biventricular pacing/CRT). These devices are used to slow HF progression and reduce the incidence of sudden death [5,10]. Their presence may complicate the placement of central venous catheters and require the involvement of an electrophysiology team for interrogation and reprogramming [11].
2
D. Mechanical circulatory support (MCS) devices
Nearly 70% of recipients in the United States require some form of life support prior to heart transplantation [12]. These include IV medications, mechanical ventilation, intra-aortic balloon pumps (IABPs), extracorporeal life support (ELS), total artificial hearts (TAH), and ventricular assist devices (VADs). IV medications, IABPs, ELS, and extracorporeal VADs are useful in temporizing a hospitalized patient with cardiogenic shock. Intracorporeal MCS devices offer the potential for discharge to home and may yield the greatest potential improvement in quality of life for patients with Class D HF who are awaiting heart transplant.
In the United States, the number of patients undergoing heart transplantation with a pre-existent VAD has risen dramatically (16% in 1999 vs. 29% in 2008) [12]. The increased use of VADs has resulted in an increase in the number of outpatients being transplanted (39% to 48%), and has in part contributed to a decline in the heart transplant waiting list mortality secondary to their use as a bridge to transplantation [12]. Six-month survival while being supported on a VAD approaches 75% [13]. However, the influence of VADs on post–heart- transplant survival is controversial, with some studies suggesting an increased 6-mo mortality after transplant, while more recent studies suggest no increase in mortality [14–17]. Confounding the task of answering these questions is the rapid evolution of the devices being used, and problems inherent in applying results from earlier pulsatile devices to more recent continuous-flow devices.
II. Cardiac transplant recipient characteristics
Between 1999 and 2008, the number of active transplant candidates in the United States declined by 32%, despite an increase of 20% between 2007 and 2008 [12]. The decline has taken place despite an increasing number of patients with Stage D HF. In 1999, UNOS modified its listing system to be two-tiered (Table 16.1), and in 2006, UNOS modified the allocation of donor hearts to expand organ sharing within geographic regions. In 2007, the median time to transplantation was 113 days. Among adults, there was an increase in the number of candidates >65 yrs (9% in 1999 vs. 14% in 2008) [12]. Among all age groups, there was an increase in congenital heart disease as a primary diagnosis (4.4% in 1999 vs. 8.9% in 2008), and a decrease in coronary artery disease (CAD) as a primary diagnosis (47% in 1999 vs. 40% in 2008) [12]. Finally, the percentage of patients classified as 1A or 1B has increased dramatically (18% in 1999 vs. 46% in 2008) [12]. The reduction in waiting list mortality and increases in Category 1A and 1B candidates may be in part due to the impact of VADs.
Table 16.1 UNOS listing criteria for heart transplantation

A. Cardiac transplantation indications
Indications for heart transplantation are listed in Table 16.2 [5,18]. Potential cardiac transplant candidates must have all reversible causes of HF excluded, and their medical management optimized.
Table 16.2 Indications for heart transplantation

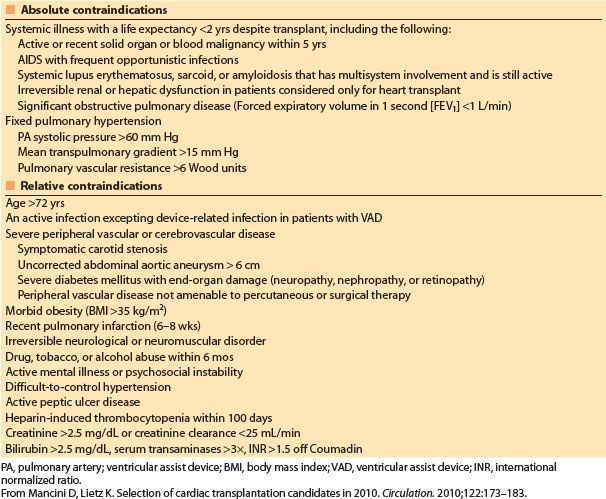
B. Cardiac transplantation contraindications
There has been a gradual relaxation in the cardiac transplantation exclusion criteria as experience with increasingly complex cases has grown [2]. Absolute exclusion criteria have been simplified (Table 16.3) [18].
Increased preoperative pulmonary vascular resistance (PVR) is predictive of early graft dysfunction and increased mortality because of an increased incidence of right heart dysfunction [18]. Methods used to quantify the severity of pulmonary HTN include calculation of PVR and the transpulmonary gradient (mean PA pressure − PCWP). At most centers, patients are not considered orthotopic cardiac transplant candidates if they demonstrate a PVR >6 Wood units or transpulmonary gradient >15 mm Hg without evidence of pharmacologic reversibility [18]. The reversibility of pulmonary HTN can be evaluated by vasodilator administration, including IV sodium nitroprusside, inhaled nitric oxide (iNO), and inhaled epoprostenol (PGI2). In patients who receive VADs, “fixed” elevated PVR may be reduced and thus improve post-transplant outcome, or qualify a previously excluded patient for heart transplant [18–22]. The only transplant options for patients with severe irreversible pulmonary HTN include heterotopic cardiac or combined heart–lung transplantation (HLT).
3
Table 16.3 Contraindications to heart transplantation
III. The cardiac transplant donor
A. Donor selection
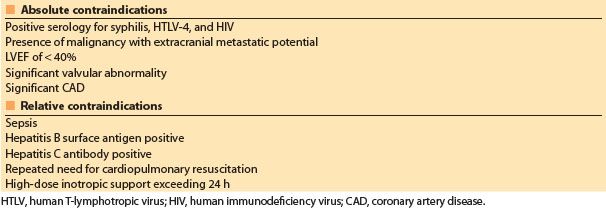
The primary factor limiting cardiac transplantation is a shortage of donors. Standard criteria for donors, first outlined in the 1980s, resulted in a paucity of donor organs relative to the number of patients who could benefit from heart transplantation. In an attempt to increase donor numbers, the criteria for cardiac organ donation have been relaxed. The so-called “marginal donor” hearts may be transplanted into borderline heart transplant candidates with good results when compared to their expected prognosis without transplantation [18,23–25]. Characteristics of marginal donors include older age (>55 yrs), the presence of CAD, donor–recipient size mismatch, history of donor drug abuse, increased ischemic times, and donor seropositivity for viral hepatitis [18,23–25]. Nonetheless, the risk of failed transplantation increases with donor age and the presence of concomitant disease [2]. Contraindications to heart donation are listed in Table 16.4.
Before a donor heart may be harvested, permission for donation must be obtained, the suitability of the heart for donation must be ascertained, and the diagnosis of brain death must be made. Initial functional and structural evaluation of the potential heart donor is made with electrocardiography and transthoracic echocardiography. Normal LV function is predictive of suitability for heart transplantation, and subsequent management of the donor may be guided by other invasive monitors such as PA catheters or serial echocardiography [24,25]. Coronary angiography may be performed on patients ≥40 yrs [26]. Donor–recipient factors such as size, ABO compatibility, and antihuman leukocyte antigen (HLA)–antibody compatibility are also be assessed. Logistic factors, including ischemic organ time, must be considered. Finally, the harvesting surgeon will directly inspect the donor heart [26].
Table 16.4 Contraindications to heart donation
B. Determination of brain death
In the United States, The Uniform Determination of Death Act defines death as either (1) the irreversible cessation of circulatory and respiratory functions or (2) irreversible cessation of all functions of the entire brain, including the brain stem. The determination of death must be made in accordance with accepted medical standards. For the diagnosis of brain death to be made, the patient’s core body temperature must be >32.5°C, and no drug with the potential to alter neurologic or neuromuscular function should be present.
C. Pathophysiology of brain death
When brain death results from severe brain injury, increased intracranial pressure results in progressive herniation and ischemia of the brain stem. Subsequent hemodynamic instability, endocrine, and metabolic disturbances disrupt homeostasis, and may render organs unsuitable for transplantation (Table 16.5).
Table 16.5 Incidence of pathophysiologic changes after brain stem death
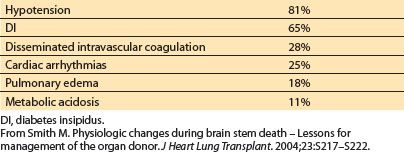
1. Cardiovascular function
In an attempt to maintain cerebral blood flow to the increasingly ischemic brain stem, blood pressure (BP) and heart rate rise. While usually transient, this adrenergically mediated “sympathetic storm” may precipitate electrocardiographic and echocardiographic findings consistent with myocardial ischemia [27–29]. Occasionally, severe systemic hypertension may persist and require management [28]. Hypotension will affect most brain-dead patients and may be refractory to pressors [28]. Hypotension may result from hypovolemia caused by traumatic blood loss, central diabetes insipidus (DI), or osmotic therapy for management of elevated intracranial pressure. Loss of sympathetic tone resulting in blunted vasomotor reflexes, vasodilatation, and impaired myocardial contractility also contributes to hypotension [28]. Noxious stimuli may induce exaggerated hypertensive responses mediated by intact spinal sympathetic reflexes that are no longer inhibited by descending pathways. Despite optimal donor support, terminal cardiac arrhythmias may occur within 48 to 72 h of brain death.
2. Endocrine dysfunction
Dysfunction of the posterior pituitary gland occurs in a majority of brain-dead organ donors. The loss of antidiuretic hormone (ADH) results in DI, which is manifested by polyuria, hypovolemia, and hypernatremia [28]. Derangements in other electrolytes including potassium, magnesium, and calcium may also occur as a result of DI. Dysfunction of the anterior pituitary has been inconsistently described, with hemodynamic and electrolyte disturbances attributable in part to loss of thyroid-stimulating hormone (TSH), growth hormone (GH), and adrenocorticotropic hormone (ACTH) [28,29]. Plasma concentrations of glucose may become variable (most often elevated) due to changes in serum cortisol levels, the use of catecholamine therapy, progressive insulin resistance, and the administration of glucose-containing fluids [29].
3. Pulmonary function
Hypoxemia resulting from lung trauma, infection, or pulmonary edema may occur following brain death. Pulmonary edema in this setting may be neurogenic, cardiogenic, or inflammatory in origin [28].
4. Temperature regulation
Thermoregulation by the hypothalamus is lost after brain death. Increased heat loss occurs as a result of an inability to vasoconstrict, along with a reduction in metabolic activity, puts brain-dead organ donors at risk for hypothermia. Adverse consequences of hypothermia include cardiac dysfunction, arrhythmias, decreased tissue oxygen delivery, coagulopathy, and cold-induced diuresis [28].
5. Coagulation
Coagulopathy may result from hypothermia, and dilution of clotting factors following massive transfusion and fluid resuscitation. For reasons that are not fully understood, disseminated intravascular coagulation occurs in approximately 28% of brain dead donors [28,29].
D. Management of the cardiac transplant donor
Post-transplant graft function is in part dependent on donor care prior to organ harvesting. Strategies for managing the brain-dead organ donors seek to stabilize the donor’s physiology so that the functional integrity of potentially transplantable organs is maintained [29].
1. Cardiovascular function
Donor systemic BP and central venous pressure (CVP) should be monitored continuously using arterial and central venous catheters [28]. Goals include a mean arterial pressure >60 mm Hg, a CVP of 6 to 10 mm Hg, urinary output >1 mL/kg/h, and a left ventricular ejection fraction (LVEF) >45% [28,30]. The initial treatment step in maintaining hemodynamic stability is aggressive replacement of intravascular volume with crystalloids, colloids, and packed red blood cells (PRBCs) if the hemoglobin concentration is less than 10 g/dL or the hematocrit is less than 30% [28,30].
If hemodynamic stability is not restored with fluid resuscitation, placement of a PA catheter, echocardiography, or continuous CO monitoring should be used to assess right- and left-sided intracardiac pressures, CO, and systemic vascular resistance (SVR) [28,30]. Use of inotropes and pressors should be guided by these additional diagnostics. Dopamine, epinephrine, and norepinephrine are commonly used for donor cardiovascular support. However, prolonged use of catecholamines at high doses should be avoided due to potential downregulation of b-receptors on the donor heart, and the negative impact this may have on graft function after cardiac transplant [27,28]. High-dose a-adrenergic receptor agonists should be used cautiously, as peripheral and splanchnic vasoconstriction may result in decreased perfusion of other potential donor organs and metabolic acidosis. An infusion of vasopressin has catecholamine-sparing effects without impairing graft function [28,30].
Brain-dead donors with hemodynamic instability refractory to fluids, catecholamines, and vasopressin have higher rates of organ procurement when hormonal therapy is added [28,30]. Thyroid hormone and methylprednisolone are part of the UNOS standard donor management protocol [30].
2. Fluid and electrolytes
Hypernatremia in the donor has been associated with higher rates of primary graft failure [28]. Aggressive treatment of DI with 1-desamino-8-D-arginine vasopressin (DDAVP) is indicated. IV fluids should be given to replace urinary losses and to maintain urine output [28]. Euglycemia (80 to 150 mg/dL) should be achieved through the use of dextrose-containing fluids or an insulin infusion [28]. Metabolic acidosis and respiratory alkalosis should be corrected, with a goal pH of 7.40 to 7.45 [28,30].
3. Pulmonary function
If lung procurement is also being considered, management of the brain-dead donor may become more complicated. The management from the standpoint of maximizing heart graft viability calls for aggressive fluid resuscitation, whereas a minimal volume strategy improves lung graft function after transplant, and recommendations for precise hemodynamic goals are different [28]. Management described here is from the standpoint of maximizing procurement of the heart.
In the absence of metabolic acidosis or alkalosis, minute ventilation should be adjusted to target a PaCO2 of 30 to 35 mm Hg [30]. The inspired oxygen concentration (FiO2) should be titrated to a PaO2 >80 mm Hg [30]. Efforts to prevent pulmonary aspiration, atelectasis, and infection are warranted. Pulmonary edema should be managed with positive end-expiratory pressure (PEEP) and diuresis.
4. Temperature
Monitoring of core temperature is mandatory as hypothermia adversely affects coagulation, cardiac rhythm, and oxygen delivery. Use of heated IV fluids, blankets, and humidifiers may prevent hypothermia.
5. Coagulation
Different transplant centers have individual guidelines for blood component therapy for management of coagulopathy. In general, component therapy should be guided by repeated donor platelet and clotting factor measurements. Generally accepted goals include an international normalized ratio (INR) of <1.5 and a platelet count of >50,000/mm3 [28]. Antifibrinolytics to control donor bleeding are not recommended due to the risk of microvascular thromboses.
E. Anesthetic management of the donor
Anesthetic management of the donor during organ harvesting is an extension of preoperative management. An FiO2 of 1 is optimal for organ viability unless the lungs are to be harvested. To decrease the possibility of oxygen toxicity in the case of donor lung harvest, the minimum FiO2 that will maintain a PaO2/FiO2 gradient of at least 300 mm Hg should be used [28]. Although intact spinal reflexes may result in hypertension, tachycardia, and muscle movement, these signs do not indicate cerebral function or pain perception. Nondepolarizing muscle relaxants may be used to prevent spinal reflex-mediated muscle movement.
4
F. Organ harvest technique
After initial dissection, the patient is fully heparinized. The perfusion-sensitive organs (i.e., kidneys and liver) are removed prior to cardiectomy. The donor heart is excised en bloc via median sternotomy after dissection of the pericardial attachments. The superior and inferior venae cavae are ligated first, allowing exsanguination. The aorta is cross-clamped and cold cardioplegia administered. The aorta and pulmonary arteries are transected, leaving the native donor arterial segments as long in length as possible. Finally, the pulmonary veins are individually divided after lifting the donor organ out of the thoracic cavity. Most donor hearts are currently preserved with specialized cold colloid solutions (e.g., University of Wisconsin solution) and placed in cold storage at 2°C [26]. When this technique is used, optimal myocardial function after transplantation is achieved when the donor heart ischemic time is less than 4 h [26].
IV. Surgical techniques for cardiac transplantation
A. Orthotopic cardiac transplantation
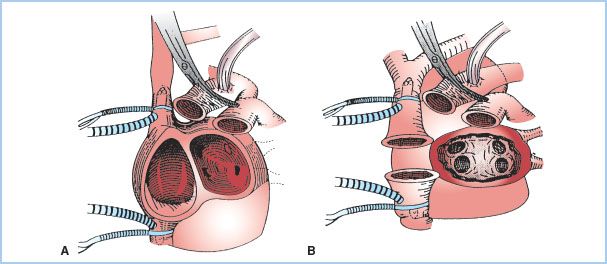
Over 98% of cardiac transplants performed are orthotopic. The recipient is placed on standard cardiopulmonary bypass (CPB) and, if present, the PA catheter withdrawn into the superior vena cava. The femoral vessels are often selected for arterial and venous CPB cannulation in patients undergoing repeat sternotomy. Otherwise, the distal ascending aorta is cannulated and bicaval cannulae with snares placed, completely excluding the heart from the native circulation. The aorta and pulmonary arteries are then clamped and divided. Depending on the implantation technique (Fig. 16.3), either both native atria or a single left atrial cuff containing the pulmonary veins is preserved. The native atrial appendages are discarded because of the risk of postoperative thrombus formation.
The donor heart is inspected for the presence of a patent foramen ovale. If patent, it is surgically closed, as right-to-left interatrial shunting and hypoxemia may occur in the presence of elevated right-sided pressures following transplantation. The donor and recipient left atria are anastomosed first, followed by the right atria, or cavae when a bicaval anastomotic technique is chosen. The subsequent order of anastomoses varies depending on the donor heart ischemic time and the experience of the surgeon. The donor and recipient aortas are joined and the aortic cross-clamp removed with the patient in Trendelenburg to decrease air embolism. After completion of the PA anastomosis and placement of temporary epicardial pacing wires, the heart is deaired and the patient weaned from CPB.
Figure 16.3 Surgical techniques for cardiac transplantation. A: Biatrial technique. The donor heart is anastomosed to the main bulk of the recipient’s native right and left atria. B: Bicaval technique. The donor heart left atrium is anastomosed to a single left atrial cuff, including the pulmonary veins, in the recipient. (From Aziz TM, Burgess MI, El Gamel A, et al. Orthotopic cardiac transplantation technique: A survey of current practice. Ann Thorac Surg. 1999;68:1242–1246, with permission.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








