KEY POINTS
1. Preoperatively respiratory function should be assessed in three related but independent areas: Respiratory mechanics, gas exchange, and cardiopulmonary interaction.
2. The most useful preoperative predictor of difficult endobronchial intubation is the chest film.
3. The most important predictor of PaO2 during one-lung ventilation is the PaO2 during two-lung ventilation in the lateral position before one-lung ventilation.
4. Absolute indications for lung isolation include purulent secretions, massive pulmonary hemorrhage, bronchopleural fistula, blebs, and bullae (blood, pus, and air).
5. Iatrogenic injury has been estimated to occur in 0.5 to 2 per 1,000 cases using double-lumen tubes.
6. The rapidity of the fall in PaO2 after the onset of one-lung ventilation is an indicator of the risk of subsequent desaturation.
7. Because of the risk of increased shunt and pulmonary edema in the dependent lung, no volume should be given for theoretical third-space losses during thoracotomy.
8. Desaturation during bilateral lung procedures is particularly a problem during the second period of one-lung ventilation.
9. The postoperative mortality following pneumonectomy is 17% in patients who develop arrhythmias versus 2% in those without this complication.
10. During induction of general anesthesia in patients with an anterior mediastinal mass, airway obstruction is the most common and feared complication.
11. Hemoptysis in a patient with a PA catheter must be assumed to be caused by perforation of a pulmonary vessel by the catheter until proven otherwise. The mortality rate may exceed 50%.
12. In a patient with pulmonary hypertension, abrupt cessation of treatment with a prostacyclin analog can result in potentially catastrophic rebound pulmonary hypertension.
13. For transplantation of CMV-negative donors and recipients, leukocyte-poor filters should be used to reduce exposure to CMV during transfusion of blood and blood products. Likewise, to avoid human leukocyte antigen alloimmunization, leukocyte-poor blood is necessary for transplant candidates.
14. There may be an increase in the dose of nondepolarizing neuromuscular blocking agents required in posttransplantation patients receiving azathioprine.

I. Preoperative assessment
A. Overview. Advances in anesthetic management, surgical techniques, and perioperative care have expanded the envelope of patients now considered to be operable. The principles described apply to all types of pulmonary resections and other chest surgery. In patients with malignancy, the risk/benefit ratio of canceling or delaying surgery pending other investigation/therapy is always complicated by the risk of further spread of cancer during any interval before resection.
A patient with a “resectable” lung cancer has a disease that is still local or locoregional in scope and can be encompassed in a plausible surgical procedure. An “operable” patient is one who can tolerate the proposed resection with acceptable risk.
1. Risk assessment. It is the anesthesiologist’s responsibility to use the preoperative assessment to identify patients at elevated risk and then to use that risk assessment to stratify perioperative management and focus resources on the high-risk patients to improve their outcome. This is the primary function of the preanesthetic assessment.
2. Initial and final assessments. Commonly, the patient is initially assessed in a clinic and often not by the member of the anesthesia staff who will administer the anesthesia. The actual contact with the responsible anesthesiologist may be only 10 to 15 min before induction. It is necessary to organize and standardize the approach to preoperative evaluation for these patients into two temporally disjoint phases: The initial (clinic) assessment and the final (day-of-admission) assessment.
3. “Lung-sparing” surgery. Postoperative preservation of respiratory function has been shown to be proportional to the amount of functioning lung parenchyma preserved. To assess patients with limited pulmonary function, the anesthesiologist must understand these surgical options in addition to conventional lobectomy and pneumonectomy.
Prethoracotomy assessment naturally involves all of the factors of a complete anesthetic assessment: Past history, allergies, medications, and upper airway. The major cause of perioperative morbidity and mortality in the thoracic surgical population is respiratory complications. Atelectasis, pneumonia, and respiratory failure occur in 15% to 20% of patients. Cardiac complications, such as arrhythmia and ischemia, occur in 10% to 15% of the thoracic population.
1
B. Risk stratification
1. Assessment of respiratory function. The best assessment of respiratory function comes from a history of the patient’s quality of life. An asymptomatic American Society of Anesthesiologists (ASA) Class I or II patient with full exercise capacity does not need screening cardiorespiratory testing. Assess respiratory function in three related but independent areas: Respiratory mechanics, gas exchange, and cardiopulmonary interaction. These three factors give the “three-legged stool” of prethoracotomy respiratory assessment (Fig. 26.1).
Figure 26.1 “Three-legged” stool of prethoracotomy respiratory assessment. ppo, predicted postoperative; FEV 1, Forced expiratory volume in one second; MVV, Maximum voluntary ventilation; RV/TLC, Residual volume/Total lung capacity; FVC, Forced vital capacity; VO2 max, Maximum Oxygen consumption; SpO2, Oxygen saturation by pulse oximetry; DLCO, Diffusing capacity for carbon monoxide; PaO2, Arterial partial pressure of oxygen; PaCO2, Arterial partial pressure of carbon dioxide.
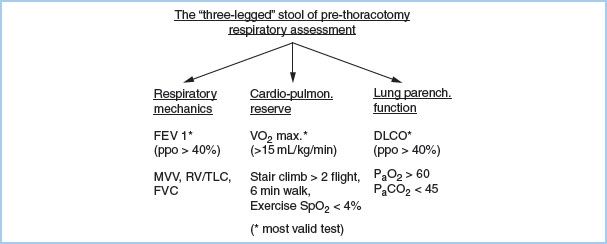
a. Lung mechanics. The most valid single test [1] for post-thoracotomy respiratory complications is the predicted postoperative forced expiratory volume in 1 s (ppoFEV1%), which is calculated as follows:
ppoFEV1% = preop. FEV1% × (1-% functional lung tissue removed/100)
Consider the right upper and middle lobes combined as approximately equivalent to each of the other three lobes and the right lung 10% larger than the left lung.
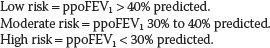
b. Pulmonary parenchymal function. Traditionally, arterial blood gas (ABG) data such as PaO2 less than 60 mm Hg or PaCO2 greater than 45 mm Hg have been used as cutoff values for pulmonary resection. Cancer resections now have been successfully done or even combined with volume reduction in patients who do not meet these criteria, although they remain useful as warning indicators of increased risk. The most useful test of the gas exchange capacity of the lung is the diffusing capacity for carbon monoxide (DLCO). DLCO correlates with the total functioning surface area of alveolar– capillary interface. A ppoDLCO less than 40% correlates with both increased respiratory and cardiac complications [2].
c. Cardiopulmonary interaction. The traditional test in ambulatory patients is stair climbing. The ability to climb three flights or more is associated with decreased mortality. Formal laboratory exercise testing is currently the “gold standard” for assessment of cardiopulmonary function. The maximal oxygen consumption VO2 max is the most valid exercise predictor of post-thoracotomy outcome. An estimate of VO2 max can be made by dividing the distance walked in meters in 6 min (6MWT) by 30 (i.e., 450 m/30 = 15 mL/kg/min):
Low risk = VO2 max > 20 mL/kg/min.
Moderate risk = VO2 max = 15 to 20 mL/kg/min.
High risk = VO2 max < 15 mL/kg/min.
d. Ventilation–perfusion scintigraphy is particularly useful in pneumonectomy patients and should be considered for any patient who has ppoFEV1 less than 40%. Assessments of ppoFEV1, DLCO, and VO2 max can be upgraded if the lung region to be resected is nonfunctioning.
e. Split-lung function studies. These tests have not shown sufficient predictive validity for universal adoption in potential lung resection patients.
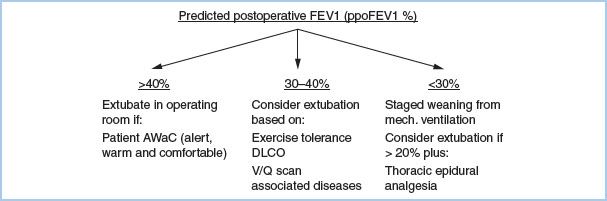
f. Combination of tests (Fig. 26.2). If a patient has ppoFEV1 greater than 40%, it should be possible for the patient to be extubated in the operating room at the conclusion of surgery, assuming the patient is alert, warm, and comfortable (“AWaC”). If ppoFEV1 is greater than 30% and exercise tolerance and lung parenchymal function exceed the increased risk thresholds, then extubation in the operating room should be possible depending on the status of associated diseases. Patients with ppoFEV1 20% to 30% and favorable predicted cardiorespiratory and parenchymal function can be considered for early extubation if thoracic epidural analgesia is used.
Figure 26.2 Post-thoracotomy anesthetic management. FEV1, Forced expiratory volume in 1 second; ppo, Predicted postoperative; DLCO, Diffusing capacity for carbon monoxide; V/Q, Ventilation/Perfusion.
2. Intercurrent medical conditions
a. Age. For patients older than 80 yrs, the rate of respiratory complications (40%) is double that expected in a younger population, and the rate of cardiac complications (40%), particularly arrhythmias, is nearly triple. The mortality from pneumonectomy (22% in patients older than 70 yrs), particularly right pneumonectomy, is excessive.
b. Cardiac disease
(1) Ischemia. Pulmonary resection is generally regarded as an intermediate-risk procedure for perioperative ischemia. Beyond the standard history, physical examination, and electrocardiogram, routine screening testing for cardiac disease does not appear to be cost effective for all prethoracotomy patients. Noninvasive testing is indicated in patients with active cardiac conditions (unstable ischemia, recent infarction, decompensated heart failure, severe valvular disease, significant arrhythmia), multiple clinical predictors of cardiac risk (stable angina, remote infarction, previous congestive failure, diabetes, renal insufficiency, or cerebrovascular disease), or in the elderly. Timing of lung resection surgery after a myocardial infarction is always a difficult decision. Limiting the delay to 4 to 6 wks in a medically stable and fully investigated and optimized patient seems acceptable.
(2) Arrhythmia. Atrial fibrillation is a common complication (10% to 15%) of pulmonary resection surgery. Factors correlating with an increased incidence of arrhythmia are the amount of lung tissue resected, age, intraoperative blood loss, and intrapericardial dissection. Prophylactic digoxin does not prevent these arrhythmias, but diltiazem may.
c. Chronic obstructive pulmonary disease (COPD). Assessment of the severity of COPD is based on FEV1% predicted, as follows—Stage I: Greater than 50%; Stage II: 35% to 50%; and Stage III: Less than 35%. The following factors in COPD need to be considered.
Respiratory drive. Many Stage II or III COPD patients have an elevated PaCO2 at rest. It is not possible to differentiate “CO2 retainers” from nonretainers on the basis of history, physical examination, or spirometry. These patients need an ABG preoperatively. Supplemental oxygen causes the PaCO2 to increase in CO2 retainers by a combination of decreased respiratory drive and increased dead space.
Nocturnal hypoxemia. COPD patients desaturate more frequently and severely than normal patients during sleep. This is due to the rapid/shallow breathing pattern that occurs during rapid eye movement sleep.
Right ventricular (RV) dysfunction. Cor pulmonale occurs in 40% of adult COPD patients with FEV1 less than 1 L and in 70% with FEV1 less than 0.6 L. COPD patients who have resting PaO2 less than 55 mm Hg and those who desaturate to less than 44 mm Hg with exercise should receive supplemental home oxygen. The goal of supplemental oxygen is to maintain PaO2 at 60 to 65 mm Hg. Pneumonectomy candidates with ppoFEV1 less than 40% should have transthoracic echocardiography to assess right heart function. Elevation of right heart pressures places these patients in a very high-risk group.
3. Preoperative therapy of COPD. The four treatable complications of COPD that must be actively sought and therapy begun at the initial prethoracotomy assessment are atelectasis, bronchospasm, chest infection, and pulmonary edema. Patients with COPD have fewer postoperative pulmonary complications when a perioperative program of chest physiotherapy is initiated preoperatively. Pulmonary complications are decreased in thoracic surgical patients who are not smoking versus those who continue to smoke up until the time of surgery.
4. Lung cancer considerations. At the time of initial assessment, cancer patients should be assessed for the “4 M’s” associated with malignancy: Mass effects, Metabolic abnormalities, Metastases, and Medications. Prior use of medications that can exacerbate oxygen-induced pulmonary toxicity, such as bleomycin, should be considered (Table 26.1).
Table 26.1 Anesthetic considerations in lung cancer patients (the “4 M’s”)

5. Postoperative analgesia. The risks and benefits of the various forms of post-thoracotomy analgesia should be explained to the patient at the time of initial preanesthetic assessment. Potential contraindications to specific methods of analgesia should be determined, such as coagulation problems, sepsis, and neurologic disorders. If the patient is to receive prophylactic anticoagulants and it is elected to use epidural analgesia, appropriate timing of anticoagulant administration and neuraxial catheter placement need to be arranged. American Society of Regional Anesthesia guidelines suggest an interval of 2 to 4 h before or 1 h after catheter placement for prophylactic heparin administration. Low-molecular-weight heparin precautions are less clear, but an interval of 24 h before catheter placement is recommended.
6. Premedication. Avoid inadvertent withdrawal of drugs that are being taken for concurrent medical conditions (bronchodilators, antihypertensives, β-blockers). For esophageal reflux surgery, oral antacid and H2-blockers are routinely ordered preoperatively. Mild sedation, such as an intravenous (IV) short-acting benzodiazepine, is often given immediately before placement of invasive monitoring lines and catheters. In patients with copious secretions, an anti-sialagogue (e.g., glycopyrrolate 0.2 mg) is useful to facilitate fiberoptic bronchoscopy (FOB) for positioning of a double-lumen tube (DLT) or bronchial blocker (BB).
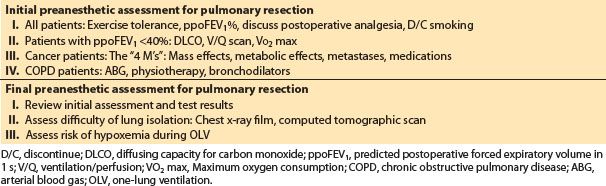
7. Final preoperative assessment. The final preoperative anesthetic assessment is made immediately before the patient is brought to the operating room. Review the data from the initial prethoracotomy assessment (Table 26.2) and the results of tests ordered at that time. Two other concerns for thoracic anesthesia need to be assessed: (i) The potential for difficult lung isolation and (ii) the risk of desaturation during one-lung ventilation (OLV).
Table 26.2 Summary of preanesthetic assessment
2
a. Assessment of difficult endobronchial intubation. The most useful predictor of difficult endobronchial intubation is the chest x-ray film. Clinically important tracheal or bronchial distortions or compression from tumors or previous surgery can be usually detected on plain chest films. Distal airway problems not detectable on the plain x-ray film may be visualized on chest computed tomographic (CT) scans. These abnormalities often will not be mentioned in a written or verbal report from the radiologist or surgeon. The anesthesiologist must examine the chest image before placing a DLT or BB.
3
b. Prediction of desaturation during OLV. It is possible to determine patients who are most at risk for desaturation during OLV for thoracic surgery [3]. The factors that correlate with desaturation during OLV are listed in Table 26.3. The most important predictor of PaO2 during OLV is the PaO2 during two-lung ventilation in the lateral position before OLV. The proportion of perfusion or ventilation to the non-operated lung on preoperative ventilation/perfusion (V/Q) scans also correlates with the PaO2 during OLV. The side of the thoracotomy has an effect on PaO2 during OLV. With the left lung being 10% smaller than the right lung, there is less shunt when the left lung is collapsed. The degree of obstructive lung disease correlates in an inverse fashion with PaO2 during OLV. Patients with more severe airflow limitation on preoperative spirometry tend to have a better PaO2 during OLV. This is related to the development of auto positive end-expiratory pressure (PEEP) during OLV in the obstructed patients.
Table 26.3 Factors that correlate with an increased risk of desaturation during one-lung ventilation

Stratifying the perioperative risks allows the anesthesiologist to develop a systematic focused approach to these patients at the time of initial contact and immediately before induction, which can be used to guide anesthetic management (Fig. 26.2).
II. Intraoperative management
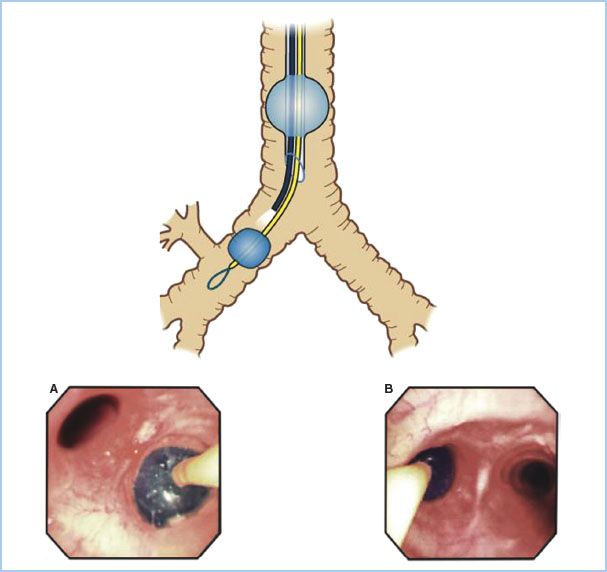
A. Lung separation. There are three basic options for lung separation: Single-lumen endobronchial tubes (EBTs), DLTs (left- or right-sided) (see Figs. 26.3 and 26.4), and BBs. The second half of the twentieth century has seen refinements of the DLT from that of Carlens to a tube specifically designed for intraoperative use (Robertshaw) with larger, D-shaped lumens and without a carinal hook. Current disposable polyvinyl chloride DLTs have incorporated high-volume/low-pressure tracheal and bronchial cuffs. Recently, there has been a revival of interest in BBs due to several factors: New blocker designs (see Figs. 26.5 and 26.6) [4] and greater familiarity of anesthesiologists with fiberoptic placement of BBs (see Fig. 26.7).
Figure 26.3 The optimal position of a left-sided double lumen endotracheal tube. A: Unobstructed view of the entrance of the right mainstem bronchus as seen from the tracheal lumen. B: Take-off of the right-upper lobe bronchus with the three segments. C: Unobstructed view of the left-upper and left-lower bronchus as seen from the bronchial lumen. (Reproduced from Campos J. Lung isolation. In: Slinger P, ed. Principles and Practice of Thoracic Anesthesia. New York, NY: Springer; 2011, with kind permission of Springer Science + Business Media.)
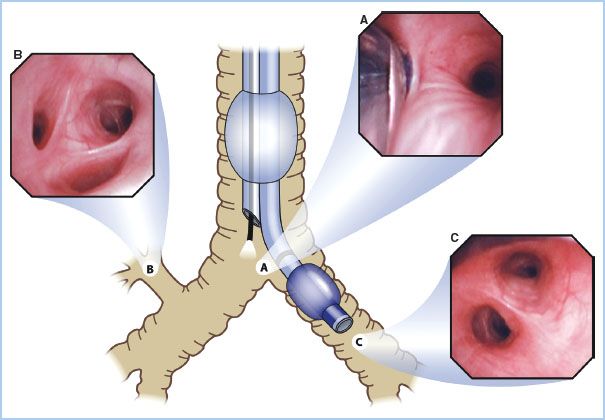
Figure 26.4 Optimal position of a right-sided double lumen endotracheal tube as seen with a fiberoptic bronchoscope. A: View of the right upper lobe bronchus seen through the ventilating side slot of the bronchial lumen. B: View of the carina showing the left mainstem bronchus and the bronchial lumen in the right mainstem bronchus seen from the tracheal lumen. (Reproduced from Campos J. Lung isolation. In: Slinger P, ed. Principles and Practice of Thoracic Anesthesia. New York, NY: Springer; 2011, with permission of Springer Science + Business Media.)
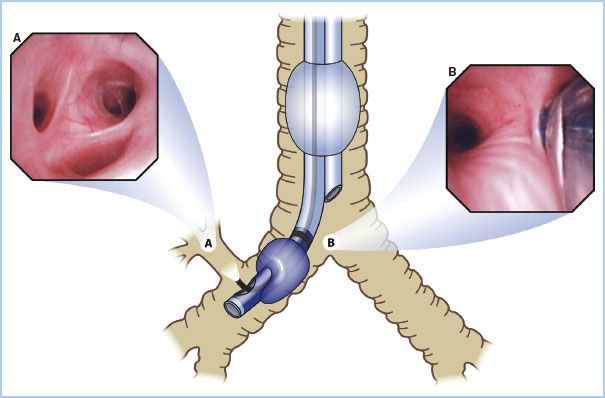
Figure 26.5 Three independent endobronchial blockers currently available in North America. Left: The Cohen tip-deflecting endobronchial blocker (Cook Critical Care, Bloomington, IN, USA), which allows anesthesiologists to establish one lung ventilation by directing its flexible tip left or right into the desired bronchus using a control wheel device on the proximal end of the blocker in combination with fiberoptic bronchoscopic (FOB) guidance. Middle: The Fuji Uniblocker (Fuji Corp., Tokyo, Japan). It has a fixed distal curve that allows it to be rotated for manipulation into position with FOB guidance. Unlike its predecessor, the Univent blocker, the Uniblocker is used with a standard endotracheal tube. Right: The wire-guided endobronchial blocker (Arndt bronchial blocker; Cook Critical Care) introduced in 1999. It contains a wire loop in the inner lumen; when used as a snare with a fiberoptic bronchoscope, it allows directed placement. The snare is then removed, and the 1.4 mm lumen may be used as a suction channel or for oxygen insufflation. (Reproduced from Campos J. Lung isolation. In: Slinger P, ed. Principles and Practice of Thoracic Anesthesia. New York, NY: Springer; 2011, with kind permission of Springer Science + Business Media.)
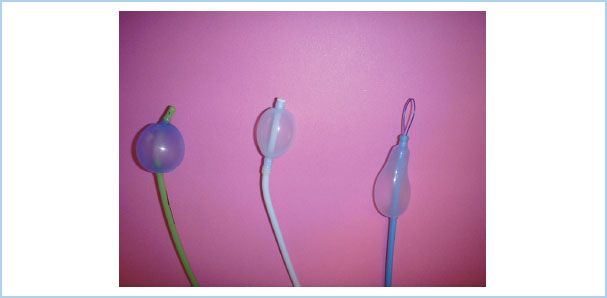
Figure 26.6 The recently introduced Arndt spherical endobronchial blocker cuff (Cook Critical Care, Bloomington, IN, USA). Some clinicians prefer to use the spherical cuff for right-sided surgery versus the original elliptical cuff because of the short length of the right mainstem bronchus. (Reproduced from Campos J. Lung isolation. In: Slinger P, ed. Principles and Practice of Thoracic Anesthesia. New York, NY: Springer; 2011, with kind permission of Springer Science + Business Media.)
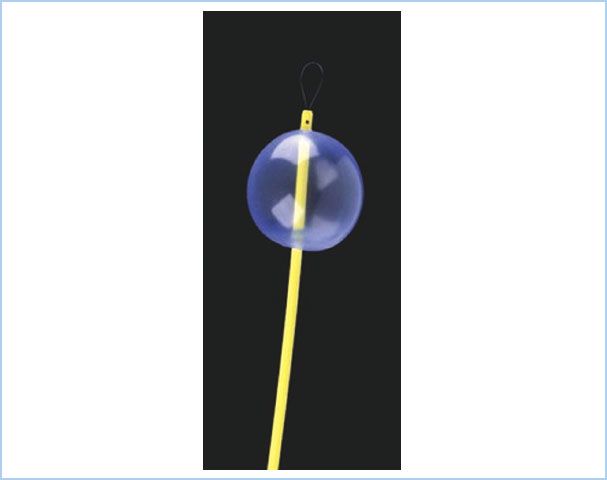
Figure 26.7 Diagram (top): Placement of an Arndt blocker through a single-lumen endotracheal tube in the right mainstem bronchus with the fiberoptic bronchoscope. Photographs (bottom): Optimal position of an endobronchial blocker in the right (A) or left (B) mainstem bronchus as seen through a fiberoptic bronchoscope. (Reproduced from Campos J. Lung isolation. In: Slinger P, ed. Principles and Practice of Thoracic Anesthesia. New York, NY: Springer; 2011, with kind permission of Springer Science + Business Media.)
1. Indications for lung separation. Absolute indications for lung isolation include purulent secretions, massive pulmonary hemorrhage, and bronchopleural fistula, blebs, and bullae (blood, pus, and air). More commonly, lung separation is provided intraoperatively to facilitate surgical exposure.
4
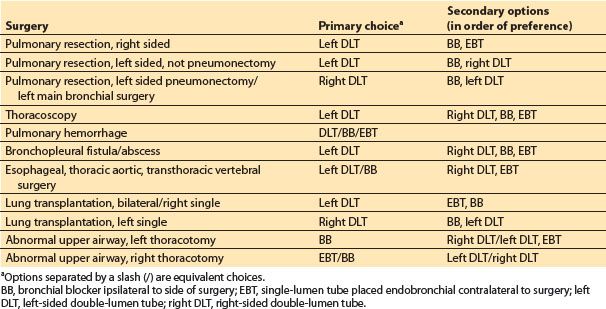
2. Techniques of lung separation. The optimal methods for lung isolation are listed in Table 26.4. Because it is impossible to describe one technique as best in all indications for OLV, the various indications are considered separately.
Table 26.4 Selection of airway device for lung isolation
a. Elective pulmonary resection, right-sided. The first choice is a left DLT. The widest margin of safety in positioning is with left DLTs. With blind positioning, the incidence of malposition can exceed 20% but is correctable in virtually all cases by fiberoptic adjustment. There is continuous access to the nonventilated lung for suctioning, fiberoptic monitoring of position, and continuous positive airway pressure (CPAP). There are two possible alternatives. (i) Single-lumen EBT: A standard 7.5 mm diameter, 32 cm long endotracheal tube (ETT) can be advanced over an FOB into the left main stem bronchus. (ii) Univent tube or BB: The BB can be placed external to or intraluminally with an ETT.
b. Elective pulmonary resection, left-sided
(1) Not pneumonectomy. There is no obvious best choice between a BB and a left DLT. Use of a left DLT for a left thoracotomy can be associated with obstruction of the tracheal lumen by the lateral tracheal wall and subsequent problems with gas exchange in the ventilated lung. A right DLT is an alternate choice.
(2) Left pneumonectomy. When a pneumonectomy is foreseen, a right DLT is the best choice. A right DLT will permit the surgeon to palpate the left hilum during OLV without interference from a tube or blocker in the left main stem bronchus. The disposable right DLTs currently available in North America vary greatly in design, depending on the manufacturer (Mallinckrodt, Rusch, Kendall). The Mallinckrodt design, currently, is the most reliable. All three designs include a ventilating side slot in the distal bronchial lumen for right upper lobe ventilation. If left lung isolation is impossible despite extremely high pressures in the right DLT bronchial cuff, a Fogarty catheter can be passed into the left main bronchus as a BB. As an alternative, there is no clear preference between a left DLT or BB. These all require repositioning before clamping the left main stem bronchus.
c. Thoracoscopy. Lung biopsies, wedge resection, bleb/bullae resections, and some lobectomies can be done using video-assisted thoracoscopic surgery (VATS). During open thoracotomy, the lung can be compressed by the surgeon to facilitate collapse before inflation of a BB. A left DLT is preferred for thoracoscopy of either hemithorax because it gives more rapid deflation of the nonventilated lung.
d. Pulmonary hemorrhage. Instances of life-threatening pulmonary hemorrhage can occur due to a wide variety of causes, such as aspergillosis, tuberculosis, and pulmonary artery (PA) catheter trauma. The primary risk for these patients is asphyxiation, and first-line treatment is lung isolation and suctioning the lower airways. Lung isolation can be with a DLT, BB, or single-lumen EBT, depending on availability and the clinical circumstances (see Section 24 III-M). Tracheobronchial hemorrhage from blunt chest trauma usually resolves with suctioning; only rarely is lung isolation necessary.
e. Bronchopleural fistula. The anesthesiologist is faced with the triple problem of avoiding tension pneumothorax, ensuring adequate ventilation, and protecting the healthy lung from the fluid collection in the involved hemithorax. Management depends on the site of the fistula and the urgency of the clinical situation. For a peripheral bronchopleural fistula in a stable patient, a BB may be acceptable. For a large central fistula and in urgent situations, the most rapid and reliable method of securing one-lung isolation and ventilation is a DLT. In life-threatening situations, a DLT can be placed in awake patients with direct FOB guidance.
f. Purulent secretions (lung abscess, hydatid cysts). Lobar or segmental blockade is ideal. Loss of lung isolation in these cases is not merely a surgical inconvenience but may be life threatening. Univent tubes can be used for lobar blockade. A secure technique in these cases is the combined use of a BB and a DLT.
g. Nonpulmonary thoracic surgery. Thoracic aortic and esophageal surgeries require OLV. Because there is no risk of ventilated-lung contamination, a left DLT and a BB are equivalent choices.
h. Bronchial surgery. An intrabronchial tumor, bronchial trauma, or bronchial sleeve resection during a lobectomy requires that the surgeon have intraluminal access to the ipsilateral main stem bronchus. Either a single-lumen EBT or a DLT in the ventilated lung is preferred.
i. Unilateral lung lavage, independent lung ventilation, and lung transplantation are all best accomplished with a left DLT.
3. Upper airway abnormalities. It is occasionally necessary to provide OLV in patients who have abnormal upper airways due to previous surgery or trauma or in patients who are known for unanticipated difficult intubations. There are four basic options for these patients: (i) Fiberoptic-guided intubation with a DLT; (ii) secure the airway with an ETT and then use a “tube exchanger” to place a DLT; (iii) use a BB; and (iv) use an uncut single-lumen tube as an EBT.
The optimal choice will depend on the patient and the operation. At all times it is best to maintain spontaneous ventilation and to do nothing blindly in the presence of blood or pus. Awake FOB intubation with a DLT requires thorough topical anesthesia of the airway. It is important when using a tube exchanger to have a second person perform a direct laryngoscopy to expose as much of the glottis as possible during the tube change. Direct laryngoscopy decreases the angles between the oropharynx and trachea and reduces the chance of trauma to the airway from the DLT. The video-laryngoscopes (e.g., glidescope) are useful for this.
BBs are often the best choice for some of these patients. If the ET tube is too narrow to easily accommodate both a bronchoscope and a BB, the BB can be introduced through the glottis independently external to the ET tube with fiberoptic guidance. Bilateral BBs can be used for bilateral resections or the same blocker can be manipulated from side to side. Bilateral single-lumen EBTs or BBs can be used for lung isolation in patients with tracheal fistulas, trauma, or other abnormalities in the region of the carina. Smaller DLTs (32, 28, and 26 Fr) are available, but they will not permit passage of an FOB of the diameter commonly available to monitor positioning (3.5 to 4.0 mm). An ETT designed for microlaryngoscopy (5 to 6 mm inner diameter [ID] and greater than 30 cm long) can be used as an EBT, with FOB positioning. If the patient’s trachea can accept a 7.0 mm ETT, a Fogarty catheter (8 Fr venous thrombectomy catheter with a 10 mL balloon) can be passed through the ETT via an FOB adapter for use as a BB.
4. Chest trauma. It is common in both open and closed chest trauma to have some hemoptysis from alveolar hemorrhage. The majority of these cases can be managed without lung isolation after bronchoscopy and suction. The majority of the deaths in these patients are due to their other injuries and not from airway hemorrhage or air embolus. Lung isolation may be helpful in some of these cases, but if resources and time are limited the priority must be the resuscitation of the patient.
5
5. Avoiding iatrogenic airway injury. Iatrogenic injury has been estimated to occur in 0.5 to 2 per 1,000 cases with DLTs.
a. Examine the chest x-ray film or CT scan, which can help predict the majority of difficult endobronchial intubations.
b. Use an appropriate size tube. Too small tube will make lung isolation difficult. Too large a tube is more likely to cause trauma. Useful guidelines for DLT sizes in adults are as follows:
(1) Females’ height < 1.6 m (63 inch): 35 Fr (possibly 32 Fr if <1.5 m).
(2) Females >1.6 m: 37 Fr.
(3) Males <1.7 m (67 inch): 39 Fr (possibly 37 Fr if <1.6 m).
(4) Males >1.7 m: 41 Fr.
c. Depth of insertion of DLT. Tracheobronchial dimensions correlate with height. The average depth at insertion, from the teeth, for a left DLT is 29 cm in an adult and varies ±1 cm for each 10 cm of patient height above/below 170 cm.
d. Avoid nitrous oxide. Nitrous oxide 70% can increase the bronchial cuff volume from 5 to 16 mL intraoperatively.
e. Inflate the bronchial cuff/blocker only to the minimal volume required for lung isolation and for the minimal time. This volume is usually less than 3 mL. Inflating the bronchial cuff does not stabilize the DLT position when the patient is turned to the lateral position.
(1) Endobronchial intubation must be done gently and with fiberoptic guidance if resistance is met. A significant number of case reports are from cases of esophageal surgery, where the elastic supporting tissue may be weakened and predisposed to rupture from DLT placement.
6. Other complications of lung separation
a. Malpositioning. Initial malpositioning of DLTs with blind placement can occur in greater than 30% of cases. Verification and adjustment with FOB immediately before initiating OLV is mandatory because these tubes will migrate during patient positioning. Malpositioning after the start of OLV due to dislodgment is more of a problem with BBs than DLTs.
b. Airway resistance. The resistance from a 37 F DLT exceeds that of a no. 9 Univent by less than 10% over the range of airflows seen with spontaneous ventilation. These flow resistances both are less than that of an 8.0 mm ID ETT but exceed that of a 9.0 mm ETT. For short periods of postoperative ventilation and weaning, airflow resistance is not a problem with a DLT.
7. The ABCs of lung separation will always apply:
a. Know the tracheobronchial anatomy [5].
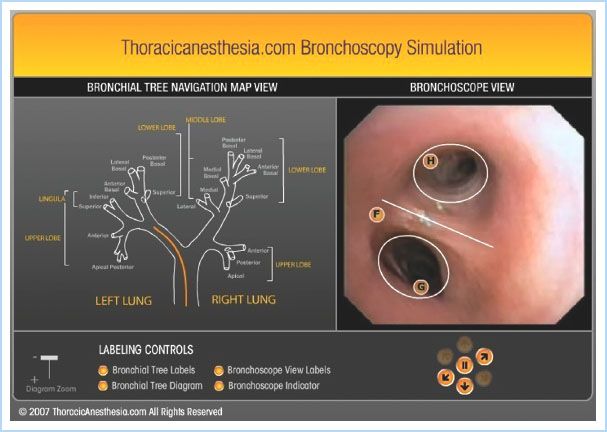
b. Use the fiberoptic bronchoscope (see Fig. 26.8) [6].
Figure 26.8 The free online bronchoscopy simulator at www.thoracicanesthesia.com. The user can navigate the tracheobronchial tree using real-time video by clicking on the lighted directional arrows under the “Bronchoscopic view” (right). Clicking on the labels on the “Bronchoscopic view” gives details of the anatomy seen. The process is aided by the “Bronchial Tree Navigational Map” (left), which shows the simultaneous location of the bronchoscope as the orange line in the airway. (Reproduced from Campos J. Lung isolation. In: Slinger P, ed. Principles and Practice of Thoracic Anesthesia. New York, NY: Springer; 2011, with kind permission of Springer Science + Business Media.)
c. Look at the chest x-ray film and CT scan in advance.
B. Positioning. The majority of thoracic procedures are performed with the patient in the lateral position, but, depending on the surgical technique, a semisupine or semiprone lateral position may be used. It is awkward to induce anesthesia in the lateral position; thus, monitors will be placed and anesthesia will be usually induced in the supine position and the anesthetized patient will then be repositioned for surgery. It is possible to induce anesthesia in the lateral position. This may rarely be indicated with unilateral lung diseases, such as bronchiectasis or hemoptysis, until lung isolation can be achieved. However, even these patients will have to be repositioned and the diseased lung turned to the nondependent side. Due to the loss of venous vascular tone in the anesthetized patient, it is not uncommon to see hypotension when the patient is turned to or from the lateral position.
All lines and monitors will have to be secured during position change and their function reassessed after repositioning. The anesthesiologist should take responsibility for the head, neck, and airway during position change and must be in charge of the operating team to direct repositioning. It is useful to make an initial “head-to-toe” survey of the patient after induction and intubation, checking oxygenation, ventilation, hemodynamics, lines, monitors, and potential nerve injuries. This survey must be repeated after repositioning. It is nearly impossible to avoid some movement of a DLT or BB during repositioning. The patient’s head, neck, and EBT should be turned en bloc with the patient’s thoracolumbar spine. The margin of error in positioning EBTs or blockers is often so narrow that even small movements can have significant clinical implications. EBT/blocker position and adequacy of ventilation must be rechecked by auscultation and FOB after patient repositioning.
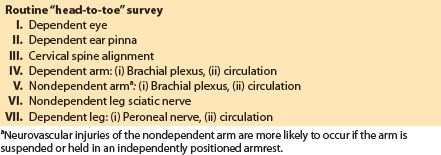
1. Neurovascular complications. The brachial plexus is the site of the majority of intraoperative nerve injuries related to the lateral position. The brachial plexus is fixed at two points: Proximally by the transverse process of the cervical vertebrae and distally by the axillary fascia. This two-point fixation plus the extreme mobility of neighboring skeletal and muscular structures make the brachial plexus extremely liable to injury. The patient should be positioned with padding under the dependent thorax to keep the weight of the upper body off the dependent arm brachial plexus. This padding will exacerbate the pressure on the brachial plexus if it migrates superiorly into the axilla. It is useful to survey the patient from the side of the table immediately after the patient is turned to ensure that the entire vertebral column is aligned properly.
The dependent leg should be slightly flexed with padding under the knee to protect the peroneal nerve lateral to the proximal head of the fibula. The nondependent leg is placed in a neutral extended position and padding placed between it and the dependent leg. The dependent leg must be observed for vascular compression. Excessively tight strapping at the hip level can compress the sciatic nerve of the nondependent leg. A “head-to-toe” protocol to monitor for possible neurovascular injuries related to the lateral decubitus position is given in Table 26.5.
Table 26.5 Avoiding neurovascular injuries specific to the lateral position
2. Physiologic changes in the lateral position
a. Ventilation. Significant changes in ventilation develop between the lungs when the patient is placed in the lateral position. The compliance curves of the two lungs are different because of their difference in sizes. The lateral position, anesthesia, paralysis, and opening the thorax all combine to magnify these differences between the lungs.
In a spontaneously breathing patient, the ventilation of the dependent lung will increase approximately 10% when the patient is turned to the lateral position. Once the patient is anesthetized and paralyzed, the ventilation of the dependent lung will decrease 15%. The compliance of the entire respiratory system will increase once the nondependent hemithorax is open.
Applying PEEP to both lungs in the lateral position, PEEP preferentially goes to the most compliant lung regions and hyperinflates the nondependent lung without causing any improvement in gas exchange. In the lateral position, atelectasis will develop in a mean of 5% of lung volume, all in the dependent lung.
b. Perfusion. Turning the patient to the lateral position decreases the blood flow of the nondependent lung due to gravity by approximately 10% of the total pulmonary blood flow.
The matching of ventilation and perfusion will usually decrease in the lateral position compared to the supine position. Pulmonary arteriovenous shunt usually increases from approximately 5% in the supine position to 10% to 15% in the lateral position.
C. Intraoperative monitoring
1. General to all pulmonary resections. The majority are major operative procedures of moderate duration (2 to 4 h) and performed in the lateral position with the hemithorax open. Consideration for monitoring and maintenance of body temperature and fluid volume should be given to all of these cases. All cases should have standard ASA monitoring. Additional monitoring is guided by a knowledge of which complications are likely to occur (Table 26.6).
Table 26.6 Intraoperative complications that occur with increased frequency during thoracotomy
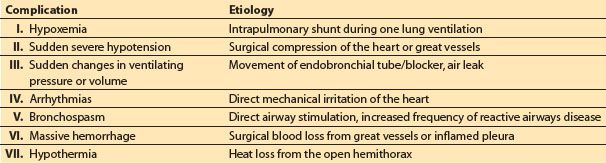
2. Specific to certain types of resection. There are complications that are more prone to occur with certain resections, such as hemorrhage from an extrapleural pneumonectomy, contralateral lung soiling with resection of a cyst or bronchiectasis, air leak hypoventilation, or tension pneumothorax with a bronchopleural fistula.
6
3. Oxygenation. Significant arterial oxygen desaturation (less than 90%) during OLV occurs in approximately 1% of the surgical population with a high FIO2 of 1.0. Pulse oximetry (SpO2) has not negated the need for direct measurement of arterial PaO2 via intermittent blood gases in the majority of thoracotomy patients. PaO2 offers a more useful estimate of the margin of safety above desaturation than SpO2. The rapidity of the fall in PaO2 after the onset of OLV is an indicator of the risk of subsequent desaturation. Measure PaO2 by ABG before OLV and 20 min after the start of OLV.
4. Capnometry. End-tidal CO2 (PetCO2) is a less reliable indicator of the PaCO2 during OLV than during two-lung ventilation and the Pa-etCO2 gradient increases during OLV. As the patient is turned to the lateral position, the PetCO2 of the nondependent lung falls relative to the dependent lung because of increased perfusion of the dependent lung and increased dead space of the nondependent lung. At the onset of OLV, the PetCO2 of the dependent lung usually falls transiently as all the minute ventilation is transferred to this lung. The PetCO2 then rises as the fractional perfusion is increased to this dependent lung by collapse and pulmonary vasoconstriction of the nonventilated lung. If there is no correction of minute ventilation, the net result will be increased baseline PaCO2 and PetCO2 with an increased gradient. Severe (greater than 5 mm Hg) or prolonged falls in PetCO2 indicate a maldistribution of perfusion between ventilated and nonventilated lungs and may be an early warning of a patient who will desaturate during OLV.
5. Invasive hemodynamic monitoring
a. Arterial line. There is a significant incidence of transient severe hypotension from surgical compression of the heart or great vessels during intrathoracic procedures. For this reason, plus the utility of intermittent ABG sampling, it is useful to have beat-to-beat assessment of systemic blood pressure during the majority of thoracic surgery cases. Exceptions are limited procedures such as thoracoscopic resections in younger/healthier patients. For most thoracotomies, placement of a radial artery catheter can be in either the dependent or nondependent arm.
b. Central venous pressures (CVPs). CVP readings obtained intraoperatively with the chest open are not completely reliable. It is our practice to routinely place CVP lines in pneumonectomy patients but not for lesser resections unless there is significant other concurrent illness. Our choice is to use a high anterior approach to the right internal jugular vein with ultrasound guidance to minimize the risk of pneumothorax for CVP access unless there is a contraindication.
c. PA catheters. The risk/benefit ratio for the routine use of PA catheters for pulmonary resection surgery favors their use only in certain specific cases, such as patients with life-threatening coexisting disease and/or patients undergoing particularly extensive procedures. Several recently developed systems of noninvasive cardiac output monitoring may prove equally as useful for this purpose (e.g., Flowtrac®, Edwards Lifesciences, Irvine, CA, USA).
6. FOB. Significant malpositions of left-sided or right-sided DLTs that can lead to desaturation during OLV are often not detected by auscultation or other traditional methods of confirming placement. Positioning of DLTs or BBs should be confirmed after placing the patient in the surgical position because a large number of the tubes/blockers migrate during repositioning of the patient.
7. Continuous spirometry. Side-stream spirometry monitors inspiratory and expiratory pressures, volume, and flow interactions during anesthesia. The adequacy of lung isolation can be monitored by breath-to-breath comparison of inspiratory and expiratory tidal volumes. This also gives a sense of the magnitude of air leaks from the ventilated lung. Changes in the position of a DLT can be detected by changes in the pressure–volume loops.
D. Anesthetic technique. Any anesthetic technique that provides safe and stable general anesthesia for major surgery can and has been used for lung resection. Many centers use combined thoracic epidural and general anesthesia for thoracic surgery.
7
1. IV fluids. Because of hydrostatic effects, excessive administration of IV fluids can cause increased shunt and lead to pulmonary edema of the dependent lung. Because the dependent lung is the lung that must carry on gas exchange during OLV, it is best to be as judicious as possible with fluid administration. IV fluids are administered to replace volume deficits and for maintenance only during lung resection anesthesia. No volume is given for theoretical third-space losses during thoracotomy (Table 26.7).
Table 26.7 Fluid management for pulmonary resection surgery

2. Nitrous oxide. Nitrous oxide/oxygen mixtures are more prone to cause atelectasis in poorly ventilated lung regions than oxygen by itself. The optimal method to prevent dependent lung atelectasis is the use of air/oxygen mixtures during both two-lung ventilation and OLV, titrating the FIO2 to avoid hypoxemia. Nitrous oxide also tends to increase PA pressures in patients who have pulmonary hypertension.
3. Temperature. Maintenance of body temperature can be a problem during thoracic surgery because of heat loss from the open hemithorax. This is particularly a problem at the extremes of the age spectrum. Most of the body’s physiologic functions, including hypoxic pulmonary vasoconstriction (HPV), are inhibited during hypothermia. Increasing the ambient room temperature and using a lower-limb forced-air patient warmer are the best methods to prevent inadvertent intraoperative hypothermia.
4. Prevention of bronchospasm. Due to the high incidence of coexisting reactive airways disease in the thoracic surgical population it is generally advisable to use an anesthetic technique that decreases bronchial irritability. This is particularly important because the added airway manipulation caused by placement of a DLT or BB is a potent trigger for bronchoconstriction. Avoid manipulation of the airway in a lightly anesthetized patient, use bronchodilating anesthetics, and avoid drugs which release histamine.
For IV induction of anesthesia, either propofol or ketamine diminish bronchospasm. For maintenance of anesthesia, propofol and/or any of the volatile anesthetics will diminish bronchial reactivity. Sevoflurane may be the most potent bronchodilator of the volatile anesthetics.
5. Coronary artery disease. Since the lung resection population is largely composed of elderly patients and smokers there is a high coincidence of coronary artery disease. This consideration will be a major factor in the choice of the anesthetic technique for most thoracic patients. The anesthetic technique should optimize the myocardial oxygen supply/demand ratio by maintaining arterial oxygenation and diastolic blood pressure while avoiding unnecessary increases in cardiac output and heart rate. Thoracic epidural anesthesia (TEA)/analgesia may help to achieve these goals.
E. Management of one-lung ventilation
1. Hypoxemia. There is an incidence of <4% of hypoxemia (arterial saturation <90%) during OLV for thoracic surgery. Hypoxemia is more likely to occur when OLV is in the supine position.
2. HPV. HPV is thought to decrease the blood flow to the nonventilated lung by 50%. The stimulus for HPV is primarily the alveolar oxygen tension (PaO2), which stimulates precapillary vasoconstriction redistributing pulmonary blood flow away from hypoxemic lung regions via a pathway involving nitric oxide (NO) and/or cyclo-oxygenase synthesis inhibition. All of the volatile anesthetics inhibit HPV. This inhibition is dose dependent. Isoflurane, sevoflurane, and desflurane cause less inhibition than other volatiles [7]. No clinical benefit has been shown for total IV anesthesia beyond that seen with isoflurane 1 MAC (minimum alveolar concentration) or less. HPV is decreased by all vasodilators such as nitroglycerin and nitroprusside. In general, vasodilators can be expected to cause a deterioration in PaO2 during OLV.
3. Cardiac output. The net effects of an increase in cardiac output during OLV tend to favor an increase in PaO2. However, elevation of cardiac output beyond physiologic needs tends to oppose HPV and may cause PaO2 to fall.
4. Ventilation during one-lung anesthesia. It is possible to improve gas exchange for selected individual patients by altering the ventilatory variables that are under the control of the anesthesiologist: Tidal volume, rate, inspiratory/expiratory ratio, PaCO2, peak and plateau airway pressures, and PEEP (Table 26.8).
Table 26.8 Ventilation parameters for one-lung ventilation
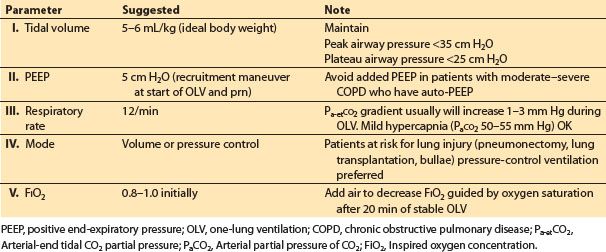
a. Respiratory acid–base status. The overall efficacy of HPV is optimal with normal pH and PaCO2.
b. Tidal volume. 5–6 mL/kg ideal body weight for OLV is a reasonable starting point. The tidal volume should be adjusted during OLV to keep the airway peak pressure less than 35 cm H2O and the plateau airway pressure less than 25 cm H2O.
c. PEEP. Most patients with either normal or supra-normal (restrictive lung disease) lung elastic recoil will benefit from low levels (5 cm H2O) of PEEP during OLV. A recruitment maneuver (e.g., static inflation to 20 cm H2O for 20 s, observing for hypotension) to the ventilated lung is useful at the start of OLV. Auto-PEEP is prone to occur in patients with decreased lung elastic recoil, such as those with emphysema. Auto-PEEP is difficult to detect using currently available anesthetic ventilators. Applied PEEP combines with auto-PEEP in an unpredictable fashion.
d. Volume control versus pressure control. Pressure-control OLV is useful in patients with severe obstructive disease and to limit airway pressure in patients with blebs, bullae, or fresh resections in the lung, and also in patients at risk of acute lung injury (pneumonectonies, lung transplantation).
e. FIO2. The FIO2 should be increased at the start of OLV to 80% to 100% and then can be decreased as tolerated over the next 20 min.
5. Treatment of hypoxemia during OLV (see Table 26.9)
In cases of severe and/or acute desaturation resume two-lung ventilation immediately, deflate the bronchial cuff or blocker, and then check the position of the DLT or BB. In cases of desaturation that have not become life-threatening:
Table 26.9 Treatment options for hypoxemia during one-lung ventilation
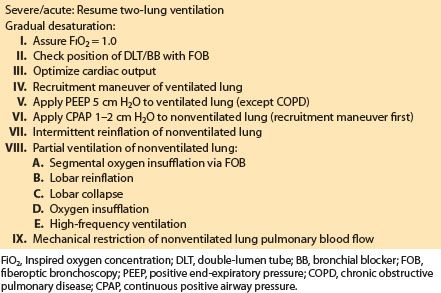
a. Increase FIO2. The first-line therapy is to increase the FIO2, which is an option in essentially all patients except those who received bleomycin or similar therapy that potentiates pulmonary oxygen toxicity.
b. PEEP. PEEP to the ventilated dependent lung will improve oxygenation in patients with normal lung mechanics and those with increased elastic recoil due to restrictive lung diseases. Apply a recruitment maneuver to the ventilated lung before application of PEEP.
c. Pharmacologic manipulations. Increasing cardiac output will result in a small but clinically useful increase in both PvO2 and PaO2 if cardiac output has decreased. Eliminating potent vasodilators, such as nitroglycerin and halothane, will improve oxygenation during OLV. Selective pulmonary vasodilators such as NO have not yet proven to be reliable.
d. CPAP. It must be applied to a fully inflated or re-inflated lung for optimal effect. When CPAP is applied to a fully inflated lung, as little as 2 to 3 cm H2O can be used. All that is required is a CPAP valve and an oxygen source. Ideally the circuit should permit variation of the CPAP level and should include a reservoir bag to allow easy reinflation of the nonventilated lung and a manometer to measure the actual CPAP supplied. Such circuits are commercially available or can be readily constructed. When the bronchus of the operative lung is obstructed or open to atmosphere, CPAP will not improve oxygenation. During thoracoscopic surgery, CPAP can significantly interfere with surgery.
e. Alternative ventilation methods. Several alternative methods of OLV, all of which involve partial ventilation of the nonventilated lung, have been described and improve oxygenation during OLV. These techniques are useful in patients who are particularly at risk for desaturation, such as those with previous pulmonary resections of the contralateral lung.
(1) Oxygen insufflation (5 L/min) for brief periods via the suction channel of an FOB to partially recruit a segment of the nonventilated lung remote from the site of surgery (e.g., a basilar segment of the lower lobe for upper lobe surgery). This is particularly useful in VATS surgery [8].
(2) Selective lobar collapse of only the operative lobe in the open hemithorax by placement of a blocker in the appropriate lobar bronchus of the ipsilateral operative lung.
(3) Differential lung ventilation by only partially occluding the lumen of the DLT to the operative lung.
(4) Intermittent reinflation of the nonventilated lung by regular re-expansion of the operative lung via an attached CPAP circuit.
(5) Conventional OLV of the nonoperative lung and high-frequency jet ventilation of the operative lung
f. Mechanical restriction of pulmonary blood flow. It is possible for the surgeon to directly compress or clamp the blood flow to the nonventilated lung. This can be done temporarily in emergency desaturation situations or definitively in cases of pneumonectomy or lung transplantation. Another technique is inflation of a PA catheter balloon in the main PA of the operative lung.
8
6. Prevention of hypoxemia. The treatments outlined as therapy for hypoxemia can be used prophylactically to prevent hypoxemia in patients who are at high risk for desaturation during OLV. Desaturation during bilateral lung procedures is particularly a problem during the second period of OLV. It is advisable to operate first on the lung that has better gas exchange. For the majority of patients this means operating on the right side first.
III. Specific procedures
A. Thoracotomy
1. Operations
a. Lobectomy. Lobectomy is the most common pulmonary resection for lung cancer. Early functional loss exceeds the amount of lung tissue resected, but function recovers over a period of 6 wks so that the final net loss of respiratory function is equivalent to the amount of functioning lung tissue excised. The recovery of pulmonary function after thoracotomy is unique because it shows a plateau with no early recovery during the first 72 h postoperatively. This period coincides with the occurrence of the majority of post-thoracotomy respiratory complications (atelectasis, pneumonia), which are the major causes of mortality after pulmonary resection. These complications are particularly associated with lobectomy and its variations, probably due to the transient dysfunction that occurs in the remaining lobe(s). The right middle lobe is particularly at risk for these complications after a right upper lobectomy and can develop torsion about its broncho-vascular pedicle or lobar bronchial kinking as it expands into the apex of the right hemithorax.
b. Sleeve lobectomy. A sleeve lobectomy is the excision of a lobe plus the adjacent segment of mainstem bronchus with bronchoplastic repair of the bronchus by end-to-end anastomosis to preserve the distal functioning pulmonary parenchyma. It is done to preserve functioning lung tissue when the tumor encroaches to less than 2 cm from the lobar bronchial orifices, precluding simple lobectomy. This procedure is usually done for right upper lobe tumors but can be used for other lobes. The anesthetic implications of this procedure are that no airway catheter (single-lumen or DLT) or BB can be placed in the ipsilateral main stem bronchus. Mucus clearance across the bronchial anastomosis may be impaired after sleeve resection, and local tumor recurrence is a problem.
c. Bi-lobectomy. In the right lung, a bi-lobectomy may be used to conserve either a functioning upper or lower lobe when the tumor extends across the lobar fissure or for malignancies involving the bronchus intermedius (the portion of the right main stem bronchus distal to the right upper lobe orifice). The complication rate is slightly higher than for a simple lobectomy but is less than for a pneumonectomy. The incidence of cardiac dysrhythmias increases postoperatively versus lobectomy, whereas the incidence of respiratory complications remains the same. The residual lobe cannot completely fill the hemithorax, and all patients will have a degree of pneumothorax that can be expected to resolve gradually.
d. Pneumonectomy. Complete removal of the lung is required when a lobectomy or its modifications is not adequate to remove the local disease and/or ipsilateral lymph node metastases. Atelectasis and pneumonia occur after pneumonectomy as they do after lobectomy but may be less of a problem because of the absence of residual parenchymal dysfunction on the operative side. The mortality rate after pneumonectomy exceeds that for lobectomy because of complications that are more likely with pneumonectomy.
(1) Postpneumonectomy pulmonary edema. The syndrome presents clinically with dyspnea and an increased alveolar–arterial oxygen gradient on the second or third postoperative day [9]. Radiologic changes precede clinical symptoms by approximately 24 h. The factors that are known about this syndrome are listed in Table 26.10. This syndrome has such a high case of fatality rate (greater than 50%) that it represents a large portion of mortality after lung resection. Excessive perioperative administration of IV crystalloids or colloids can exacerbate this syndrome. There is no evidence that judicious amounts of fluids cause this problem. The residual nonoperated lung has an increased pulmonary capillary permeability after pneumonectomy that is not seen in the nonoperated lung after lobectomy. The cause of this increased permeability may be related to surgical lymphatic damage, capillary stress injury from increased flow, or increased airway pressure and hyperinflation of the ventilated lung during OLV. The majority of lobectomies have the potential to become a pneumonectomy, so caution is needed with fluids in most thoracic cases.
Table 26.10 Postpneumonectomy pulmonary edema

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








