KEY POINTS
1. In adult CHD, a clinically relevant classification of lesions into three categories is useful: (1) Complete surgical correction, (2) partial surgical correction or palliation, and (iii) uncorrected CHD.
2. Over 1 million patients with congenital heart disease have reached adulthood in the United States.
3. Improvements in surgical techniques have allowed 90% of children with CHD to survive to adulthood with relatively normal functionality.
4. Ventricular and atrial arrhythmias are extremely common in adult CHD, accounting for nearly 50% of emergency hospitalizations.
5. Adult CHD patients have an incidence of pulmonary artery hypertension (PAH) as high as 10%.
6. Patients with PAH have a high surgical mortality rate (4% to 24%).
7. For both cyanotic (right-to-left shunts) and left-to-right shunts, there are general principles that impact anesthetic management.
8. Patients with complicated residual lesions requiring medium- to high-risk surgery should be managed at centers of excellence with physicians and staff trained in adult congenital disease.

I. Introduction
In 1938, Robert Gross performed the first ligation of a patent ductus arteriosus (PDA), thus initiating a major advance in congenital heart surgery and paving the way for development of modern surgical techniques [1]. Major improvements followed, with significant improvements in mortality throughout the 70s and 80s, leading to a greater survival. In 2000, the 32nd Bethesda Conference report generated from the American College of Cardiology indicated that approximately 85% of patients operated on with congenital heart disease (CHD) survive to adulthood [2]. It was estimated that 800,000 patients with adult congenital heart disease (ACHD) were in the United States in 2000. This report highlighted the importance of an emerging problem in our health care system. The issue is how to develop a model for seamless transition of care of patients presently cared for at pediatric heart centers who now must move into the adult population and adult hospitals. There has been a widespread call for an increased number of physicians capable of providing continuity of care for these patients in an outpatient setting, as well as during the perioperative period. This section will focus on the specific issues facing the patient with ACHD as they enter the perioperative period as it relates to the anesthesiologist.
II. Epidemiology
A. Defining ACHD: Attempts to establish prevalence and even mortality data for ACHD depend on the defining characteristics of which patients to include. A strict definition of ACHD was proposed by Mitchell et al., “a gross structural abnormality of the heart or intrathoracic great vessels that is actually or possibly of functional significance” [3]. This definition excludes persistent left-sided vena cava, abnormalities of major arteries, and in addition excludes bicuspid aortic valve (AV) disease, mitral valve prolapse, and the like [4].
B. Classification: A pathologic categorization scheme divides patients into categories of great complexity, moderate complexity, and simple CHD [5,6] (Table 15.1). These categories are particularly helpful in neonatal disease, as well as to establish prevalence data. In ACHD, a different categorization approach may be more clinically relevant and will be utilized later in this section. These three categories are listed below [7]:
1
1. Complete surgical correction
a. Examples include repaired atrial septal defect (ASD), ventricular septal defect (VSD), and PDA without hemodynamic sequelae.
2. Partial surgical correction or palliation
a. Examples include palliative repairs such as Fontan, tetrology of Fallot (ToF), and transposition of great arteries (TGA) (Mustard repair), leaving hemodynamic or physiologic compromise.
Table 15.1 Adult congenital heart disease classification [7]
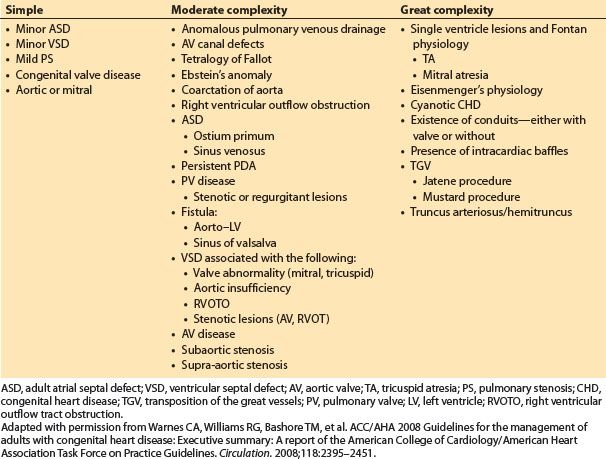
3. Uncorrected CHD
a. Examples include minor ASD, minor VSD, Ebstein’s anomaly, or undiagnosed ACHD due to limited health care access as child.
C. Prevalence
1. The actual number of adult patients with congenital heart defects is difficult to obtain; however, recent estimates suggest that over 1 million patients have reached adulthood in the United States, with an additional 8,500 corrected each year [4,8]. Significant improvements in surgical techniques have allowed many patients (>90% of children with CHD) to survive to adulthood, and maintain relatively normal function [2,4,8].
2
2. Select populations
a. A recent estimate from Canada reported 11.89 cases per 1,000 children and 4.09 cases per 1,000 adults with CHD. Selecting out patients with complex ACHD (Table 15.1) reduces these estimates to 1.45 per 1,000 children and 0.38 per 1,000 adult cases.
3
b. ACHD becomes a significant issue in certain populations such as obstetrics where patients with CHD now represent the majority (60% to 80%) of obstetric patients with cardiac disease. This population is in general young and healthy, so it makes sense that as patients with CHD reach childbearing age, they begin to represent a higher proportion of patients in this group with cardiac disease.
3. Survival data
a. It is estimated that 96% of newborns who survive the first year will reach the age of 16 [4].
b. Median expected survival has increased significantly since 2000, with current estimates placing the median age of death for ACHD at 57 yrs [4].
D. Health care system considerations
1. The ACC/AHA 2008 guidelines for ACHD highlight the fact that the pediatric cardiology centers have significant infrastructure to support patients with CHD, but that this is largely lacking in the adult health care system. This includes access to physicians with training in ACHD, as well as advanced practice nursing, case management, and social workers familiar with the needs of these patients [9]. These guidelines echo the recommendations made by the Bethesda Conference, as well as the Canadian Cardiovascular Society Consensus Conference statements from 2010 [2,10–15].
2. Overall recommendations taken from these reports suggest a focus on improvement in ACHD health care delivery through the following:
a. Improved transition clinics for adolescents approaching adulthood
b. Outreach programs to educate patients and families of key issues related to their disease
c. Enhanced education of adult caregivers trained in ACHD management
d. Coordination of health care delivery through regional centers of excellence
e. Development of primary-care physicians with ready referral access to these regional centers of excellence
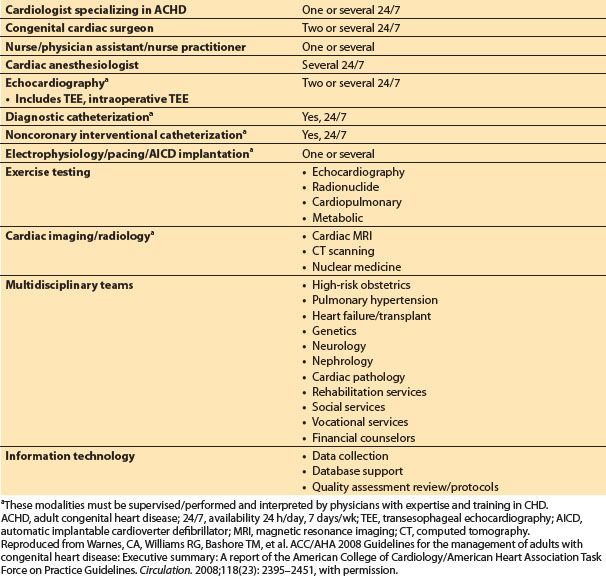
3. Centers of excellence
a. The services and provider requirements for such centers are summarized in Table 15.2, taken from the 2008 ACC/AHA guidelines. Key areas with physicians specializing in ACHD are indicated.
Table 15.2 Summary of qualifications for regional centers of excellence in adult congenital heart disease
III. What are the key anesthetic considerations in ACHD?
To evaluate the ACHD patient prior to surgery, the anesthesiologist must gain an understanding of the patient’s medical history, current functional status, state of surgical repair, and overall health. Key items are discussed below.
A. History: Obtaining a thorough and accurate surgical and medical history is critical, however challenging, as only half of patients with ACHD are able to correctly describe their diagnosis [16]. Patients with ACHD have varying functional capacities which may make evaluation of true cardiac capacity more challenging.
B. Signs and symptoms of ACHD: Some generalized exam findings that may indicate ACHD include the following [17]:
1. Continuous heart murmurs: There are relatively few acquired cardiac diseases producing a continuous type of murmur.
2. Right bundle branch block (RBBB): This can occur in the general population; however, if found in conjunction with a continuous murmur, this may indicate a congenital defect.
3. Evidence of cyanosis without existing pulmonary disease
4. The above findings should trigger an echocardiographic study prior to surgical care.
C. How can you assess the perioperative risk for ACHD patients?
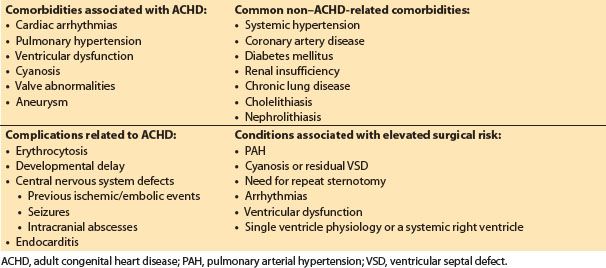
Anesthetic evaluation should focus on predicting risk of surgery in this patient population. Some key prognostic indicators for outcomes in ACHD surgery (both cardiac and noncardiac) include the presence of the below-listed factors [7,9,16,18] (Table 15.3):
Table 15.3 Common medical concerns in patients with ACHD [16]
1. Pulmonary arterial hypertension (PAH)
2. Cyanosis or residual VSD
3. Need for reoperation (cardiac surgery)
4. Arrhythmias
5. Ventricular dysfunction
6. Single ventricle physiology or a systemic right ventricle
IV. What common sequelae are associated with ACHD?
In contrast to the neonate with CHD, ACHD patients begin to acquire additional medical comorbidities that should be considered in management planning. Common comorbidities in this patient population are listed in Table 15.3, and preoperative evaluation should take these into account. Cardiac arrhythmias, pulmonary hypertension, ventricular dysfunction, cyanosis (or residual VSD), valve abnormalities, and aneurysms represent some of the key comorbid conditions commonly associated with ACHD that have serious management considerations and result in overall increased perioperative risk [4]. Obtaining a detailed history on the degree of involvement of these issues will enable adequate planning in management. Two of the most common and critical areas (arrhythmias and pulmonary hypertension) will be addressed here.
4
A. Arrhythmias: Ventricular and atrial arrhythmias are extremely common in ACHD patients accounting for nearly 50% of emergency hospitalizations [7]. The type of rhythm disturbance depends primarily on the lesion and method of surgical repair. Tables 15.4 and 15.5 divide the bradyarrhythmias from tachyarrhythmias by lesion type.
Table 15.4 Tachyarrhythmias associated with ACHD [4,7,20]
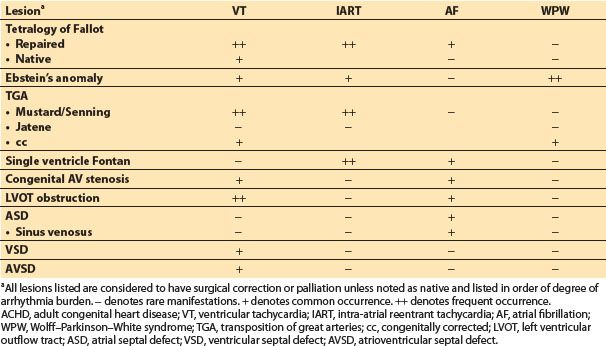
Table 15.5 Bradyarrhythmias associated with ACHD [4,7,20]

1. In general, patients who fall in the moderate to complex categories are at higher risk for arrhythmias. Tetralogy of Fallot (Fig. 15.1) and Fontan lesions carry an extremely high arrhythmia burden [19,20]. In addition, any patient with a ventricular repair or patch is at high risk for ventricular rhythm disturbances, while those patients with atrial repairs, atrial baffles, etc., are likely to develop atrial arrhythmias [20].
Figure 15.1 Macro–re-entrant VT in tetralogy of Fallot. A: An autopsy specimen of repaired tetralogy with the anterior RV surface opened to reveal the VSD patch and the patch-augmented RVOT (the outflow patch in this case is transannular). A hypothetical re-entry circuit is traced onto this image (black arrows), with the superior portion of the loop traveling through the conal septum (upper rim of the VSD). B: Actual electroanatomic map of sustained VT from an adult tetralogy patient, showing a nearly identical circuit. The propagation pattern is shown by the black arrows and is reflected by the color scheme (red > yellow > green > blue > purple). A narrow conduction channel was found between the rightward edge of the outflow patch scar (gray area) and the superior rim of the tricuspid valve. A cluster of radiofrequency applications at this site (pink dots) closed off the channel and permanently eliminated this VT circuit. LV, left ventricle; MPA, main pulmonary artery; TV, tricuspid valve. (Reused with permission from Walsh EP, Cecchin F. Congenital heart disease for the adult cardiologist: Arrhythmias in adult patients with congenital heart disease. Circulation. 2007;115:534–545.)
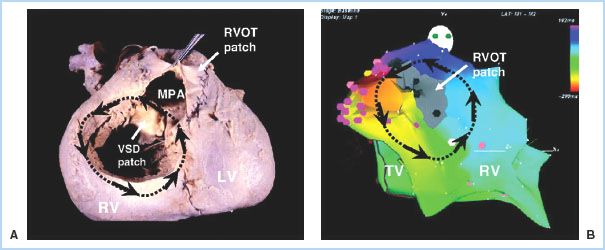
2. Patients with right-sided lesions have a higher likelihood of developing arrhythmias, although the morbidity/mortality results are similar between right- and left-sided lesions [21].
3. Patients with either ASD or VSD can have interruption in the normal conduction pathways or abnormal variants such as duplicate AV nodes (Fig. 15.2), leading to re-entrant arrhythmias [20].
Figure 15.2 Representation of twin AV nodes with a Mönckeberg sling. The cardiac anatomy in this sketch includes a large septal defect in the AV canal region, shown in a right anterior oblique projection. Both an anterior and a posterior AV node are depicted (each with its own His bundle) along with a connecting “sling” between the two systems. This conduction arrangement can produce two distinct non–pre-excited QRS morphologies (depending on which AV node is engaged earliest by the atrial activation wave front), and a variety of re-entrant tachycardias. (Redrawn from Walsh EP, Cecchin F. Congenital heart disease for the adult cardiologist: Arrhythmias in adult patients with congenital heart disease. Circulation. 2007;115:534–545.)

4. Management
a. Antiarrhythmic medical therapy remains the mainstay for most patients, although results are often suboptimal in many cases such as intra-atrial re-entrant tachycardia (IART), despite the use of potent agents such as amiodarone [20].
b. Ablative procedures: Recent advances in electrophysiology have allowed significant improvements in management of these rhythm disturbances. Electrophysiologists are able to map out the conduction pathways in the heart, and ablate malignant tracts (Figs. 15.1 and 15.3). This is most useful for atrial arrhythmias, with short-term success rates nearing 90% [20]. Long-term outcomes following ablation are less promising and not widely reported. de Groot et al. reported a 59% recurrence after the initial ablation, with the location of the recurrent pathway being different for all but one patient. At 5 yrs, 58% of patients were in sinus rhythm and 33% of the initial population were maintained on antiarrhythmic drug therapy [22]. Electrophysiologic testing and ablative procedures are considered a Class I recommendation for patients with known rhythm disturbances [9].
Figure 15.3 Electroanatomic map of an IART circuit involving the anterolateral surface of the right atrium in a patient with a previous Fontan operation (cavopulmonary connection). A detailed anatomic shell was generated for the ablation procedure by merging high-resolution computed tomography data with real-time data gathered from the 3D mapping catheter. The propagation pattern for the IART circuit is shown by black arrows and is also reflected by the color scheme (red > yellow > green > blue > purple). The critical component of the circuit appeared to be a narrow conduction channel through a region of scar (central gray area). A cluster of radiofrequency applications (maroon dots) was placed at the entrance zone to this narrow channel and permanently eliminated this IART circuit. LPA, left pulmonary artery; RPA, right pulmonary artery; RA, right atrium; JXN, junction; LAT, lateral. (Reused with permission from Walsh EP, Cecchin F. Congenital heart disease for the adult cardiologist: Arrhythmias in adult patients with congenital heart disease. Circulation. 2007;115:534–545.)

c. Implantable devices: For patients at risk for ventricular arrhythmias, automatic implantable cardiac defibrillators (AICD) can offer a life-saving modality and are a class II recommendation for ACHD patients [9]. While ventricular tachycardia (VT) is rare in the first and second decades, it becomes increasingly prevalent as the patient ages, with those patients with a history of ventricular intervention being at highest risk [20]. VT circuits can develop a macro–re-entrant characteristic similar to the atrial IART (Fig. 15.3). ToF patients have a high risk, and a careful history should be obtained, inquiring about symptoms and any outpatient studies. Patients at risk for bradyarrhythmias often will have a pacemaker in place, which should be interrogated and sensitivity limits adjusted for the surgical procedure [18].
(1) Anesthetic management: The most recent practice advisory (2011) for patients with implanted cardiac devices recommends that AICD’s be disabled prior to surgery to prevent inadvertent defibrillation due to electrocautery; however, this is specific to procedures where electromagnetic interference is likely [18]. If the AICD is disabled, it is imperative that the patient be placed on continuous monitoring, with external defibrillation pads in place, and that the device be enabled again in the PACU.
(2) Complex patients: Actual placement of AICD leads into the heart in patients with complex lesions is often difficult, if not impossible [20]. Presence of abnormal venous return, surgically created shunts or baffles, as well as scarring from previous surgery can make adequate placement challenging. Occasionally, patients will require an open surgical approach to place epicardial pacing/defibrillation leads, although this procedure often carries risk as well due to scarring and reoperative concerns (see Section IX below).
5
B. Pulmonary Arterial Hypertension (PAH): PAH is defined as a mean pulmonary pressure greater than 25 mm Hg at rest or 30 during exercise [23]. ACHD patients have an incidence of PAH in up to 10% of patients, with Eisenmenger’s syndrome (ES) being present in approximately 1% [4]. The etiology of the PAH typically falls in the World Health Organization category I or II. Group I is PAH due to primary PAH but includes congenital shunts, and group II is due to pulmonary venous hypertension (i.e., disease due to valve disorders, volume excess, and LV dysfunction).
6
1. Surgical risk: PAH patients are high-risk surgical candidates. Published series demonstrate a range of surgical mortalities from a low of 4% to a high of 24% depending on disease severity and surgical procedure [24]. Surgical and anesthetic risk should be clearly stated to the patient, especially for an elective case. Patients with ES should be considered higher-risk candidates and extreme care should be taken in managing these cases. See Section XII.B.2.
a. Hemodynamic spiral: Acute deterioration is possible as RV failure causes reduced pulmonary blood flow, leading to hypoxia which subsequently increases the pulmonary vascular resistance (PVR). The elevated PVR ultimately leads to increased strain on the RV. This initiates a catastrophic hemodynamic chain of events where the decreased RV stroke volume decreases LV output, and coronary blood flow to both the LV and RV decreases. The already failing RV may not be able to recover from this insult, resulting in cardiac arrest. This “death spiral” is always a potential in PAH patients; the anesthesia provider should be aware of it and take steps to prevent it [23].
2. How do you treat RV failure in the setting of PAH? Treatment of acute RV failure should focus on reducing PVR (see Section IV.B.3 below), while utilizing b-stimulating inotropic agents such as dobutamine and/or phosphodiesterase-inhibiting agents such as milrinone, as these provide inotropic support with moderate reductions in PVR (and systemic vascular resistance [SVR]). Consider using a vasopressor such as norepinephrine in the setting of systemic hypotension to increase the coronary perfusion pressure. In severe scenarios, an intra-aortic balloon pump can also be used to increase coronary perfusion pressure, thus supporting the RV [8].
3. What are some treatment modalities during surgery to reduce PVR acutely [7]?
a. Consider moderate hyperventilation (PaCO2 ~25 to 30 mm Hg) while administering 100% oxygen.
b. Use low-pressure ventilation if possible as high intrathoracic pressure can mechanically compress extra-alveolar vessels and reduce CO.
c. Utilize nitric oxide for acute reductions in PVR.
d. Consider inhaled prostanoid (iloprost) if available.
e. Intravenous (IV) magnesium sulfate may provide temporary reductions in PVR.
C. What are some general hemodynamic goals for patients with PAH [23]?
Anesthetic and hemodynamic goals for pulmonary hypertension
1. Avoid elevations in PVR: Prevent hypoxemia, acidosis, hypercarbia, and pain. Provide supplemental oxygen at all times. Consider inhaled nitric oxide (iNO) to acutely decrease PVR.
2. Maintain SVR: Decreased SVR dramatically reduces CO due to “fixed” PVR.
3. Avoid myocardial depressants and maintain myocardial contractility.
4. Maintain chronic prostaglandin therapy without altering dosage.
5. Utilize low-pressure mechanical ventilation when possible.
V. What laboratory and imaging studies are needed?
The overall goal in preoperative laboratory and imaging studies is to assist the physicians in understanding the degree of involvement of any comorbid disease.
A. Preoperative laboratory and imaging testing should be guided by degree of severity of disease. Patients with normal functional status can be treated as any adult presenting for surgery, whereas the patient with severe functional limitation due to cardiac disease warrants additional evaluation. Lab evaluation may include complete blood count, coagulation studies, and basic metabolic studies.
B. Cardiac catheterization and/or echocardiography studies are particularly helpful in symptomatic patients by providing information on structural status of the heart, functional status of the ventricles, and degree of PAH. Many patients will also have either magnetic resonance imaging (MRI) or computed tomography (CT) reconstructive imaging as part of standard surveillance, and these can add tremendous information to the history.
C. EKG should be obtained at baseline as there are often abnormalities present. This can also alert the practitioner to the presence of a pacemaker or other abnormal rhythm disturbances.
D. Chest radiography: It can be helpful to determine degree of heart and lung disease at baseline.
VI. What monitors should be used in ACHD surgery?
A. Standard ASA monitors should be employed for every case. In addition, most cases involving moderate to complex ACHD patients will utilize some degree of invasive monitoring. Some key considerations here include the following [25]:
1. Location of arterial line, if needed, should consider previous surgical procedures such as Blalock–Taussig shunts using the subclavian artery, which compromise blood flow to the ipsilateral upper extremity.
2. Central venous catheters should be reserved for the most symptomatic patients as risk of thrombus and stroke is higher.
3. Pulmonary artery (PA) catheters are often anatomically difficult or impossible to place and are seldom helpful in patients with cyanotic cardiac lesions.
4. Transesophageal echocardiography (TEE) may be the most useful real-time monitor of cardiovascular status, especially when using general anesthesia, and should strongly be considered for patients with reduced functional status for all medium- and high-risk procedures.
5. Near-infrared spectroscopy (NIRS) has been suggested as a tool to monitor both cerebral and peripheral oxygenation. The concept is that this technology helps identify changes in oxygen delivery and may be more sensitive to changes in cardiac output. See the discussion in the previous chapter on pediatric CHD.
VII. What are some general intraoperative anesthetic considerations for patients with ACHD?
While the pathologic categorization (simple, moderate, and complex) of CHD is useful, a more clinically based approach may be more useful in intraoperative planning. One scheme would be to consider patients based on surgical correction such as the following:
A. Complete surgical correction (i.e., repaired ASD, VSD, and PDA). Patients with surgically corrected lesions, as well as palliated lesions with good functional results, typically demonstrate hemodynamic stability and normal physiology. As such, these can be assumed to be very low-risk patients and managed as an otherwise healthy adult patient.
B. Partial surgical correction or palliation (i.e., Fontan, ToF, and TGA [Mustard or Senning repair]). Palliated patients with complex disease and reduced functional capacity due to the type of lesion should be managed with more concern and will be the main focus below.
C. Uncorrected lesions (i.e., minor ASD, minor VSD, and Ebstein’s anomaly). Uncorrected patients warrant thorough examination into type of lesion and current functional state, as often these are minor lesions if they have not caused any medical or functional issues into adulthood.
7
D. General approach: For both cyanotic lesions (right-to-left shunts) and left-to-right shunts, there are general concepts that will aid in careful anesthetic planning.
1. Cyanotic lesions [25]
a. Cyanotic lesions have some element of right-to-left shunt, often even after surgical repair. The degree of this shunt determines the level of cyanosis present. Caution should be taken with sedative medicines, as lowering ventilation can increase PVR and exacerbate cyanosis by increasing right-to-left shunt.
b. Right-to-left shunting reduces the uptake of inhalational anesthetics and can prolong inhalation induction. Conversely, the onset of IV induction may be hastened.
c. Nitrous oxide may elevate PA pressure and should be used cautiously.
d. Air embolus: Take extreme care to avoid an air embolus. All IV lines should be aggressively deaired and monitored during medication administration. Epidural catheter placement should use saline for loss of resistance, not air, because air into an epidural vein can cross into the systemic circulation.
e. SVR: Changes in SVR disrupt the balance between pulmonary and systemic circulations to change the shunt. All anesthetic medications should be slowly titrated to prevent rapid changes. This holds true for both regional and general anesthetics.
f. Single-shot spinal anesthetic techniques are generally contraindicated, as quick onset of spinal sympathectomy is poorly tolerated.
g. Administration of antibiotics (vancomycin), if given quickly, may reduce SVR and become clinically relevant.
h. Choice of anesthetic induction drug is not as important as the manner and vigilance used by the anesthesiologist in managing hemodynamics.
i. Clinical endpoints that might decrease PVR, such as increases in mixed venous O2 (typically via high FiO2) and modest degrees of respiratory alkalosis, are encouraged.
2. Chronic left-to-right shunting: Balance between SVR and PVR determines the shunt fraction and the direction of shunting. Chronic left-to-right shunting causes the following:
a. Excessive pulmonary blood flow leading to pulmonary edema or pulmonary hypertension. The increased pulmonary flow causes PVR increases over time, reducing left-to-right shunting, and eventually equilibration of left and right ventricular pressures. Eventually, this process results in conversion of the left-to-right shunt into a right-to-left shunt, the so-called Eisenmenger’s physiology or syndrome.
b. Once ES develops, cyanosis ensues along with variable degrees of heart failure which places patients in the highest-risk category for surgical procedures.
c. Even without Eisenmenger’s complex, these patients may experience heart failure as a result of the high RV and pulmonary blood flow, which may be as much as four times systemic blood flow.
d. SVR: Acute changes in SVR from anesthetic administration or pain can result in alteration or reversal of the shunt, leading to heart failure or cyanosis, depending upon where the patient is in her evolution from large left-to-right shunt into the right-to-left shunting of Eisenmenger’s physiology. Overall anesthetic goals should be to maintain the balance that the patient has and avoid abrupt alterations.
e. High levels of supplemental oxygen may allow for reduced PVR and worsening of the left-to-right shunt. On the other hand, hypoxemia should be prevented as this may shift the shunt to right-to-left and result in cyanosis. A fine balance must be struck when managing oxygenation for these patients.
f. Air embolus: As in cyanotic lesions, take extreme care to avoid an air embolus. Even predominant left-to-right shunts can become bidirectional, putting the patient at risk for a systemic air embolus.
g. Single-shot spinal anesthesia is contraindicated for patients who have or are approaching Eisenmenger’s physiology. Spinal anesthesia is theoretically beneficial for patients with large left-to-right shunts and normal or only slightly elevated PVR that remains far below SVR.
h. Inhalational agents: Uptake should not be affected by left-to-right shunting. Right-to-left shunting prolongs inhalation inductions, but this is rarely clinically relevant.
VIII. What is the ideal approach to postoperative management for ACHD patients?
Postoperative management should take into account all the risk factors described above in the anesthetic planning, and one should attempt to maintain the patient in the hemodynamic state to which he/she has adapted.
A. Pain management: Patients with palliated lesions often have some degree of residual shunt, or even single ventricle physiology. As such, overall cardiac performance depends to a large degree on the PVR. Attempts should be made to minimize impairment of ventilation in these patients as hypercarbia will increase PVR and potentially worsen cyanosis or increase ventricular failure in susceptible patients.
1. Regional anesthesia may be ideal for patients with significant anticipated postoperative pain as this can greatly reduce the level of systemic opioid use, thus reducing risk of respiratory complications. Laboratory evaluation of coagulation status should be obtained in any patient with a history of anticoagulant therapy prior to neuraxial interventions.
B. Arrhythmias: For patients at elevated risk (Tables 15.4 and 15.5), perioperative monitoring in a telemetry bed may be indicated if there is not an AICD in place. For patients with AICDs or pacemakers, consider postoperative interrogation of the device if there was significant electrical interference during the surgery, or if the device had a magnet applied. Additionally, if the AICD was disabled for the procedure, it is imperative that defibrillation equipment be immediately available until the device is turned back on.
C. Volume considerations: Many ACHD patients with palliated lesions have a narrow margin of error in fluid management. They can easily be pushed into heart failure with too aggressive fluid management, and conversely may develop significant reductions in cardiac output with a minimalist approach. There is not an ideal volume strategy that fits all patients, but management must be closely tailored to each patient’s physiologic status. As discussed above, invasive monitoring may not be possible in many of these patients or may not accurately reflect actual volume status, so management can be complicated. TEE use intraoperatively along with close monitoring of urine output may be the best approach in complex patients.
IX. What is the approach for patients presenting for repeat sternotomy?
A. What are the key surgical considerations in preparation for repeat sternotomy?
In patients with ACHD, the need for repeat sternotomy is often encountered as the initial challenge regarding surgical intervention. Often these patients have had multiple prior chest surgeries, increasing the degree of scarring in the pericardial space and thus making the surgical approach more demanding. Overall mortality increases with repeat sternotomy and is reported to be in the range of 3% to 6%. Re-entrant injury has been reported to greatly increase the risk in certain series and may approach 18% to 25%. However, other reports indicate no increase in mortality but a significant increase in duration of surgery [26–29]. The risk appears to correlate to increased number of sternotomies, presence of single ventricle diagnosis, and presence of an RV–PA conduit.
1. Preoperative preparation: Several preoperative variables are of importance and can prove to be quite valuable in planning a repeat sternotomy. A PA and lateral chest radiograph can be quite helpful and should always be examined prior to operative intervention. The radiographs can supply important information regarding number of sternal wires in place and their condition as well as the lateral film in particular providing clues as to the degree of distance between the posterior sternal table and the heart itself. In addition, many patients have had preoperative cardiac catheterization studies. It is always quite helpful to assess this study and the lateral images in particular to obtain an anatomic roadmap as to areas of concern regarding the repeat sternotomy. These pictures can provide much data as to what portion of the sternum may be more impacted by adhesions to cardiac structures and which sternal wires are in closest approximation to these areas of concern.
2. Cannulation options: Should there be any significant concern regarding repeat sternotomy and a high index of suspicion for injury, femoral cannulation should be considered. Preoperative discussions with the perfusion and anesthesia teams is critical to planning alternative strategies for cannulation and the decision to begin use of the CPB circuit before completing the repeat sternotomy.
3. Specifics of repeat sternotomy: Several techniques are of importance when pursuing a repeat sternotomy. Manipulation of the surgical table with anesthesia assistance can be critical in obtaining better visualization of the posterior table of the sternum as one pursues the repeat entry from below. In addition, use of specified retractors can also be of great assistance (mammary retractor) to allow for slow and sequential separation and elevation of the sternum. The goal of this portion of re-entry should be to obtain safe removal of previously placed sternal wires and separation of the sternum.
4. Lysis of adhesions: Once the repeat sternotomy is accomplished, significant lysis of adhesions is undertaken. Good communication with the team is critical during this process. The surgical goals should be to define and separate from scar tissue areas of cannulation (assuming the patient was not cannulated via femoral access before initiating the repeat sternotomy). These areas include the ascending aorta, right atrium (in single venous cannulation), and/or the superior vena cava (SVC) and inferior vena cava (IVC) (in cases of bicaval cannulation). Further dissection of the heart and possible previously placed shunts may be more safely accomplished once cannulae are in place for initiation of CPB.
5. Initiation of CPB: It is important to have all systemic to PA conduits/shunts adequately isolated and secured prior to initiation of CPB. Once bypass is begun, these connections must be ligated so that the circuit will not induce pulmonary overcirculation and systemic undercirculation.
B. What are the key anesthetic considerations in preparation for repeat sternotomy?
The majority of ACHD patients requiring cardiac intervention will require repeat sternotomy. Often these patients have had multiple prior chest surgeries, increasing the degree of scarring in the pericardial space, thus making the surgical approach more demanding. Key anesthetic considerations for repeat sternotomy revolve around preparation for possible re-entrant injury as well as increased transfusion requirements.
1. Large-bore IV access is critical in the event of re-entrant injury. Consider the patient’s vascular anatomy and evaluate any possible central venous clots/strictures as these patients may have had multiple central lines in the past. Ultrasound guidance is recommended during line placement to help evaluate vasculature. A large-bore central venous catheter (8.5 Fr introducer) in addition to one to two large-bore peripheral IV’s attached to a high-flow fluid warmer may be prudent.
2. Placement of external defibrillator patches since access to an open chest for internal defibrillation may be delayed.
3. Type-specific blood products should be available and double-checked in a cooler in the OR at incision. Many patients will have had multiple transfusions in the past, and thus may have unique antibody profiles, which can delay the type and cross process. Typically, one should have 6 to 10 units of PRBCs available.
4. Ventilation management during re-entry should be discussed with the surgical team. There is suggestion that slight hyperinflation of the lungs, using a recruitment maneuver during sternal spreading, can actually minimize re-entrant injury as it reduces venous return through the increased intrathoracic pressure, decreasing the size of the RV and reducing the risk of re-entrant injury [30].
5. Full discussion of risk should be undertaken with the surgical team before surgery. On the basis of this discussion, the surgical team may elect to cannulate the femoral vessels or perform axillary cannulation to enable emergent institution of cardiopulmonary bypass in the event of re-entrant injury.
C. What is the role of antifibrinolytic therapy?
Fibrinolysis is known to occur during cardiopulmonary bypass and is associated with increased blood loss and need for transfusions in cardiac surgery. Due to this, antifibrinolytics have been recommended in the Society of Thoracic Surgeons and Society of Cardiovascular Anesthesiologists (STS/SCA) guidelines recently updated for 2011 [31]. Aprotinin was withdrawn from the world market in 2008 due to concerns for increased mortality despite reduction in blood loss which was demonstrated in various trials [31,32]. Current STS/SCA recommendations include routine use of either aminocaproic acid or tranexamic acid for all cardiac surgeries with a typical regimen being to initiate the infusion prior to skin incision and continue throughout the operation [32–36].
X. When should ACHD patients be listed for transplantation and what are the outcomes?
A. Heart and lung transplant can be a life-saving measure for the patient who has developed severe heart failure. ACHD patients most commonly listed for transplant include patients with uncorrectable or partially palliated lesions such as those listed below [9]:
1. Single ventricle physiology with pulmonary vascular disease (heart/lung transplant)
2. Lesions associated with LV dysfunction due to pulmonary vascular disease (heart/lung or isolated lung transplant)
3. Isolated heart failure without significant pulmonary vascular disease (more common in single ventricle physiology, or transposition of the great vessels [TGV] patients treated by atrial switch procedures) (heart transplant).
4. Patients who clinically meet the metrics for transplant should have a thorough pretransplant evaluation assessing the anatomy of the patient, as well as PVR. Longstanding elevations in PVR can easily lead to right heart failure in the donor heart and must be anticipated in these patients.
a. For cases involving elevated PVR, it is recommended to take steps to avoid acute right heart failure in the transplanted donor heart. This often involves a combination of iNO and vasoactive infusions (dobutamine, milrinone) to provide inotropic support along with pulmonary vasodilation. See chapter on heart transplantation for full discussion.
B. What are the outcomes of transplant? ACHD patients make up nearly 3% of the total number of patients listed for cardiac transplantation [37]. Davies et al. investigated patients listed for transplant from 1995 to 2009. This study indicated that the ACHD patients who went on to obtain a heart transplant had a higher early mortality, possibly due to increased repeat sternotomy incidence in this group, but an equivalent long-term mortality as non-CHD patients (53% 10-yr survival in both groups).
XI. What are the specific details for managing patients with partially corrected or palliative repairs?
A. Fontan repair: Fontan palliation has been the primary surgical approach for complex lesions such as tricuspid atresia (TA), hypoplastic left heart, double inlet LV, double-outlet RV, severe AV defects, and heterotaxy syndrome [38]. Management of these lesions in both the neonate, and the adult patient, is one of the biggest challenges in anesthetic practice [39]. Survival rates are now approximately 90% at 10 yrs following palliation; thus, more and more Fontan patients may present for adult surgery [40]. This lesion is described in detail for the neonate previously, but key aspects pertaining to adult management are presented below.
1. Pathophysiology: TA creates a situation where blood must pass from the right atrium to the left atrium via an ASD, where it mixes with pulmonary venous return. Blood flow is then directed to both the PA and the aorta by various routes. Regardless of type of repair, blood flow depends entirely on the left ventricle for cardiac output [25,38]. For a more detailed review, please see the extensive discussion of adult Fontan physiology provided by Drs. Eagle and Daves in 2011 [38].
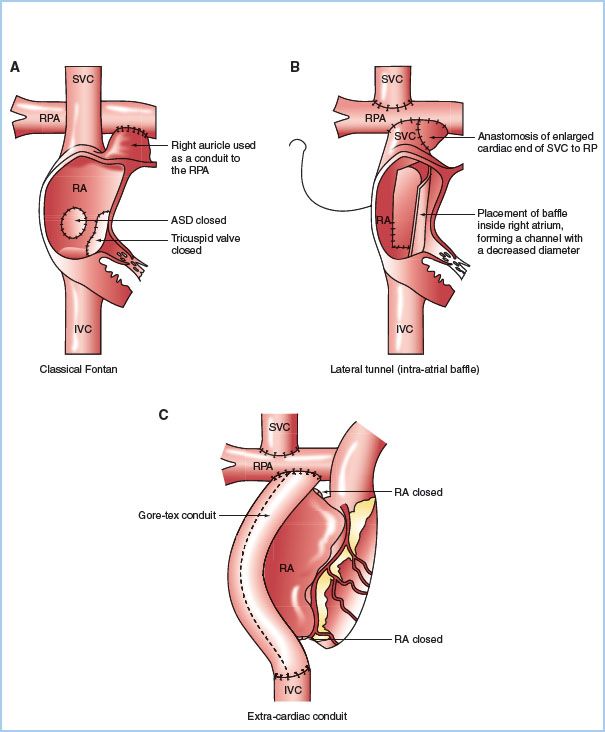
2. Surgical correction. The Fontan procedure is the definitive palliative surgical approach that creates a univentricular circulation via a cavopulmonary anastamosis. The Fontan procedure consists of creation of a classical or bidirectional Glenn shunt (SVC to PA connection), closure of the ASD, ligation of the proximal PA, and creation of a right atrial or IVC to PA connection. Multiple variations to the Fontan procedure exist (Fig. 15.4); however, the same general physiologic principles apply to most situations [25].
Figure 15.4 Fontan surgical techniques: Classical atriopulmonary connection (A), lateral tunnel (B), and extracardiac conduit (C). (Redrawn from d’Udekem, Iyengar AJ, Cochrane AD, et al. The Fontan procedure: Contemporary techniques have improved long-term outcomes. Circulation. 2007;116:I-157–I-164.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








