III.Surgical considerations
A. Technical aspects
1. CD involves the delivery of the fetus through an incision created in the maternal abdomen. For CD, either neuraxial or general anesthesia is used. Anesthetic choice depends on the urgency of the procedure and maternal comorbidities.
2. To perform a CD, the obstetrician may perform either transverse or vertical skin incision.
a. There are two types of transverse incisions:
(1) Pfannenstiel incision. An incision that is slightly curved 2 to 3 cm above the symphysis pubis with the midportion of the incision within the shaved area of the pubic hair. By using a curved incision, there is less cutting of the nerves of the anterior abdominal wall.
(2) Maylard incision. An incision that is straight and is located 3 cm below the line that joins the anterior superior iliac spines. This incision is slightly more cephalad than the Pfannenstiel incision. It also involves transection of the anterior rectus sheath and the rectus muscle bilaterally. Despite transection of the rectus muscle, this incision has been shown not to affect abdominal wall strength.
b. A vertical skin incision extends from the umbilicus to the pubic symphysis. It allows for rapid access to the uterus but is associated with a higher incidence of development of an umbilical hernia later in life.
c. Transverse incision versus vertical uterine incisions
The decision of whether to perform a transverse versus vertical incision is based solely on the expediency in which delivery must occur. Transverse incisions are less painful and are associated with a decreased risk of developing an umbilical hernia. Once the abdomen has been entered, the obstetrician must create an incision in the uterus for delivery of the infant. There are three types of uterine incisions.
(1) A low transverse incision (Kerr) is used in the majority of cases. It carries less risk of entry into the upper uterine segment and less bladder dissection. Vaginal birth with a subsequent pregnancy is possible after this incision, and it is associated with a low incidence (0.8%) of uterine rupture.8
(2) A low vertical incision involves an incision in the lower uterine segment. This incision may easily be extended upward, both deliberately and accidentally. Given this concern for accidental extension, a low vertical incision is not frequently performed. The risk of rupture with this incision (1.0%) is not as great as with a classical (vertical) uterine incision but is greater than with a low transverse incision.8
(3) A classical incision involves the upper uterine segment. This incision has a greater risk of adhesion formation and greater risk of uterine rupture or dehiscence with subsequent pregnancies (approximately 10%). A classical incision in the uterus is a contraindication to subsequent vaginal delivery.8
d. There is safety and benefit to delayed cord clamping at delivery.9 It should be considered routine in order to:
(1) Stabilize the transitional circulation, decrease the need for inotropes and blood transfusions, and reduce the risk of necrotizing enterocolitis and intraventricular hemorrhage in preterm neonates.
(2) Decrease the incidence of iron deficiency anemia and increase iron stores in term neonates.
e. After delivery of the infant, skin-to-skin contact may be initiated for the infant. Skin-to-skin contact involves the infant lying prone on the mother’s bare chest, with the infant covered by a blanket. Early contact is associated with better thermoregulation of the newborn and improved breastfeeding. Skin-to-skin contact is possible with CD in selected patients and will depend on local protocols, which vary from institution to institution.10
f. The obstetrician may or may not close the peritoneum after delivery of the infant. There is no difference in adhesion formation with or without closure of the peritoneum. Closure of the peritoneum increases surgical time.11
CLINICAL PEARL A vertical uterine incision is a contraindication to subsequent vaginal delivery.
IV.Complications of cesarean delivery
Complications of CD are either surgical or anesthesia related. However, CD can also increase the risk in subsequent pregnancies.
A. Anesthetic complications include hypotension, dyspnea, local anesthetic toxicity, total or high spinal, spinal headache, failed intubation, aspiration, awareness and recall, and death.
1. The risk of anesthetic-related maternal mortality continues to decline. Most anesthesia-related maternal deaths are preventable (see Chapter 32, Maternal Morbidity and Mortality).
a. When comparing anesthesia-related deaths from 1979–1990 to 1991–2002, anesthetic-related maternal mortality has declined 60%.12 This is attributed to the increased use of neuraxial anesthesia. Anesthetic complications account for 0.3% of all pregnancy-related deaths in the United States.13
b. The leading cause of maternal death in women receiving neuraxial blockade for CD was a high block in a recent survey of U.S. maternal mortality.12 High blocks can also be related to unrecognized intrathecal catheters as well as spinal local anesthetic administration after failed epidural anesthesia.
c. In addition, lethal infectious complications of neuraxial blockade, hypotensive cardiac arrest, reflex-mediated bradycardia, and respiratory arrest are other potential causes of anesthetic-related maternal mortality.
2. A recent report analyzing data from the Nationwide Inpatient Sample during the years 1998 through 2011 determined that cardiac arrest complicated 8.5 per 100,000 hospitalizations for delivery (99% confidence interval [CI], 7.7 to 9.3 per 100,000).14 Hemorrhage, heart failure, amniotic fluid embolism, and sepsis were identified as etiologies for the cardiac arrests. Although survival depended on the etiology of the arrest, nearly 60% of patients survived to hospital discharge.
CLINICAL PEARL High neuraxial block is a leading cause of maternal mortality. Unrecognized intrathecal catheters as well as local anesthetic administration after failed epidural anesthesia are important contributors.
B. Surgical complications
1. Intraoperative surgical complications occur in 12% to 15% of CDs.15
a. The most common complication is hemorrhage. Hemorrhage results from uterine atony, uterine lacerations, or broad ligament hematomas.
b. The incidence of transfusion for primary CD is 3.2%, with the median transfused volume being 2 units. The majority of blood for primary CD is transfused postoperatively. The incidence of transfusion for repeat CD is 2.2%, with the majority of transfusions occurring postoperatively. The median amount of blood transfused is 2 units. Risk factors for transfusion include placenta previa and preoperative anemia.16
c. The need for transfusion is not great due to the increase in blood volume accompanying pregnancy, but an atonic uterus can lose up to 2 L of blood in 5 minutes. Furthermore, obstetric blood loss is often underestimated.
d. General anesthesia for CD is associated with a higher blood loss than neuraxial anesthesia. However, this increase in blood loss is NOT associated with an increase in the need for blood transfusion.17
e. Table 13.2 lists the suggested resources that should be available for management of obstetric hemorrhage.

f. Uterine or uterocervical lacerations are the next most common complications.
g. Other complications include bladder laceration, fetal laceration, and hysterectomy.
2. Postoperative complications include anemia, fever, urinary tract infection, urinary retention, endometritis, thrombosis, ileus, and wound infection.18
CLINICAL PEARL The most common surgical complication of CD is hemorrhage (see Chapter 16, Obstetric Emergencies).
C. Subsequent pregnancy risk
1. CD increases the risk of placental abruption in subsequent pregnancies. The uterine scar from the CD leads to impaired placental perfusion, which increases the risk of placental abruption by approximately 24-fold. Placental abruption refers to separation of the placenta after 20 weeks of gestation but before the birth of the fetus.
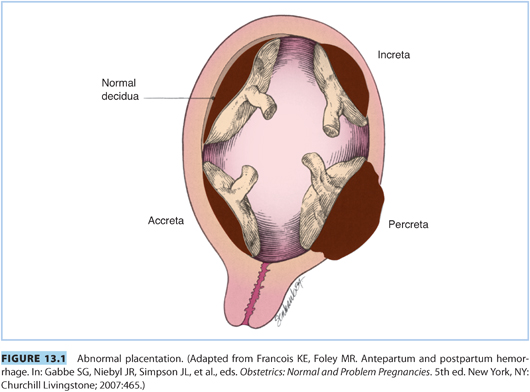
2. CD increases the risk of placenta previa in subsequent pregnancies. The uterine scar may lead to low implantation of the placenta. Placenta previa occurs when the placenta overlies the cervical os or is proximate to the internal os of the cervix. Placenta accreta is defined as a placenta that invades the uterine wall and is inseparable from it. This placental abnormality gives rise to three situations: (1) placenta accreta adheres directly to the myometrium; (2) placenta increta invades the myometrium; and (3) placenta percreta invades through the myometrium into the serosa and potentially into adjacent organs (e.g., bowel, bladder) (see Fig. 13.1). In a multicenter study of more than 30,000 patients who had CD without labor, the risk of placenta accreta was 0.2%, 0.6%, 2.1%, 2.3%, and 7.7% for women experiencing their first through sixth CDs, respectively. In patients with placenta previa in the current pregnancy, the risk of placenta accreta was 3%, 11%, 40%, 61%, and 67% for those undergoing their first through their fifth or greater CD, respectively. In patients with placenta previa and placenta accreta, massive intraoperative blood loss is common, averaging 2 to 3 L.19
CLINICAL PEARL CD increases the risk of placental abruption and placenta previa in subsequent pregnancies.
3. Cesarean deliveries that require hysterectomy to control hemorrhage are associated with significant maternal hemorrhage. Blood loss may exceed 3 L, with the majority of patients requiring intraoperative transfusion. Although it is possible to accomplish these cases with neuraxial anesthesia, general anesthesia is preferred. Early intubation removes the concern of intubation in the setting of hemodynamic instability and edema of the patient’s airway. When patients require intraoperative hysterectomy, many will develop postoperative infection, and 8% of these patients will have urologic injury. After a cesarean hysterectomy, some women may require blood vessel embolization in the interventional radiology department in order to treat persistent postoperative bleeding.
4. A prior CD does not necessitate a subsequent CD. TOLAC (which is called vaginal birth after cesarean [VBAC] if successful) is possible following a low transverse uterine incision. The risk of uterine rupture is small, approximately 0.5%. Obstetric practice patterns and resources affect the ability to offer TOLAC. In New Mexico, the number of counties offering it decreased from 100% to 41%, citing anesthesia availability, hospital and malpractice policies, malpractice costs, and obstetrician availability as the top reasons for not offering trial of labor after CD.20 Prior to 1980, it was felt that once a patient had a CD, she should always have a CD with subsequent deliveries. From 1980 to 2000, there was increased enthusiasm for TOLAC. However, this enthusiasm declined with the publication of a retrospective cohort study that showed a uterine rupture incidence of 2.5% in women with previous CD who were being induced with prostaglandins.21 Data suggest that three of five women who attempt TOLAC will be successful. However, maternal obesity, history of dystocia, and induction of labor reduce the likelihood for successful VBAC. The ACOG states that anesthesia personnel must be immediately available if a laboring parturient is attempting TOLAC. This requirement is difficult for many groups to accomplish, removing TOLAC as an option for many patients.
CLINICAL PEARL Data suggest that three out of five women who attempt TOLAC will be successful.
V.Preoperative considerations
A. Preoperative assessment
All patients who will undergo CD require a detailed history and physical examination. The physical examination must be as comprehensive as necessary but particular attention should be paid to examination of the airway and the back. According to the American Society of Anesthesiologists (ASA) Practice Guidelines for Obstetric Anesthesia, “The anesthesiologist should conduct a focused history and physical examination before providing anesthesia care.”1
CLINICAL PEARL The anesthesiologist should conduct a focused history and physical examination before providing anesthesia care.
B. Consent (see Chapter 5, Ethical and Legal Considerations in Obstetric Anesthesia)
Obtaining informed medical consent from a patient is a process by which the risks, benefits, and alternatives are explained by the anesthesia provider. The following elements are required.
1. The patient is competent to make decisions regarding her health care.
2. The anesthesia provider discloses the anesthetic risks in a noncoercive manner.
3. The patient comprehends the information.
4. The patient authorizes consent voluntarily.
C. Blood products
1. Postpartum hemorrhage is a leading cause of maternal mortality. Although there is no difference in the risk of hemorrhage in patients undergoing elective CD and uncomplicated vaginal delivery, patients undergoing CD during labor are at greater risk for hemorrhage.
2. There is no consensus about whether a type and screen or a type and cross match should be obtained prior to CD.1 Maternal history and risk of hemorrhage should guide decision making.
3. Blood should be readily available for high-risk cases (e.g., placenta accreta).
CLINICAL PEARL Patients undergoing CD during labor are at greater risk for hemorrhage.
D. Aspiration prophylaxis
1. All parturients undergoing CD should receive aspiration prophylaxis preoperatively including an H2-receptor blocker, metoclopramide, and a nonparticulate antacid.1 Aspiration prophylaxis is a recommendation of the ASA Practice Guidelines for Obstetric Anesthesia.1
2. Parturients in labor are at an increased risk for aspiration during general anesthesia. Gastric emptying is slowed with the onset of painful contractions and parenteral opioids. Epidural and intrathecal fentanyl can also impair gastric emptying. If a patient aspirates, the patient is at risk for the development of pneumonitis. In 1946, Mendelson reported 66 cases of aspiration of stomach contents during obstetric anesthesia.22 Of these patients, 5 patients aspirated solid material; 21 patients were subsequently diagnosed as having aspirated; and 40 patients aspirated liquid material. The aspiration of solid materials definitely increases the risk of aspiration pneumonitis. For liquid material, the risk of aspiration pneumonitis is dependent on the pH of the solution (increased if pH<2.5) and volume of the solution (increased if the volume is >25 mL). The administration of above mentioned aspiration prophylaxis is thought to reduce the risk for aspiration pneumonitis.23
3. “The oral intake of modest amounts of clear liquids may be allowed for uncomplicated laboring patients. The uncomplicated patient undergoing elective cesarean delivery may have modest amounts of clear liquids up to 2 h before induction of anesthesia. Examples of clear liquids include, but are not limited to, water, fruit juices without pulp, carbonated beverages, clear tea, black coffee, and sports drinks. The volume of liquid ingested is less important than the presence of particulate matter in the liquid ingested. However, patients with additional risk factors for aspiration (e.g., morbid obesity, diabetes, difficult airway) or patients at increased risk for operative delivery (e.g., nonreassuring fetal heart rate pattern) may have further restrictions of oral intake, determined on a case-by-case basis.”1
4. “Solid foods should be avoided in laboring patients. The patient undergoing elective surgery (e.g., scheduled cesarean delivery or postpartum tubal ligation) should undergo a fasting period for solids of 6–8 h depending on the type of food ingested (e.g., fat content).”1
CLINICAL PEARL Gastric emptying is slowed with the onset of painful contractions and parenteral opioids. Epidural and intrathecal fentanyl can also impair gastric emptying.
E. Supplemental oxygen
The routine administration of supplemental oxygen during CD has been questioned because there is evidence that it may be ineffective and, in some cases, detrimental because of the conversion of oxygen to free radical oxygen species. Current evidence suggests that supplementary oxygen given to healthy term pregnant women during elective CD under neuraxial anesthesia is associated with higher maternal and neonatal oxygen levels (maternal SpO2, PaO2, UaPO2, and UvPO2) and higher levels of oxygen free radicals. However, the intervention appears neither beneficial or harmful to the neonate’s short-term clinical outcome as assessed by Apgar scores.24
F. Other preparation
The ASA Practice Guidelines for Obstetric Anesthesia state that the operating room for CD must have the same equipment as for regular surgery.1 The ASA standards for basic monitoring apply to the care of patients undergoing CD. The incidence of failed intubation for CD is 1:224 general anesthetics. Whether the use of videolaryngoscopy on the incidence of failed intubation is unclear.25 A difficult airway cart as well as supplies for massive hemorrhage and malignant hyperthermia should also be readily available (see Chapter 14, Difficult Airway Management in the Pregnant Patient).
G. Intraoperative medications
1. Prophylactic antibiotics are administered to reduce the risk of endometritis, wound infections, urinary tract infections, and fever. ACOG recommends administration of a first-generation cephalosporin or other narrow-spectrum antibiotic within 1 hour of the surgical incision.26 However, a recent database review of over 1 million women who underwent CD examined rates of antibiotic use and determined that only 60% of patients received antibiotics on the day of surgery.27 The study revealed a large variation by geographic region but no influence of age, race, or insurance status.
CLINICAL PEARL A first-generation cephalosporin or other narrow-spectrum antibiotic should be administered within 1 hour of the surgical incision.
2. Vasopressor administration
a. A vasopressor must be readily available. Either phenylephrine or ephedrine is an acceptable choice.
b. The initial thought that ephedrine was the preferred agent for the treatment of hypotension during CD was based on animal models in which α-agonists decreased uterine blood flow but mixed agonists had no effect. Subsequent studies did not demonstrate an effect on uterine blood flow from α-agonists.
c. A statistically significant difference in improved umbilical arterial pH was found for phenylephrine, but the clinical importance of this small difference is uncertain. The difference is not due to an effect on uterine blood flow but because ephedrine crosses the placenta and stimulates the release of fetal catecholamines. The increase in catecholamine levels leads to an increase in oxygen consumption and an increase in lactate concentration.28
d. The effect of ephedrine on umbilical arterial pH may also be a result of genetic susceptibility in certain fetuses. Those infants who do develop an acidosis tend to have similar genetics.29
e. Phenylephrine is typically administered by continuous infusion, especially when spinal anesthesia is administered. The infusion is initiated at the time of administration of the intrathecal medications. There is no difference in umbilical arterial pH when phenylephrine is administered as a continuous infusion or as intermittent boluses. With a continuous infusion, there is a lower incidence of maternal nausea and vomiting.30
f. Either phenylephrine or ephedrine is an acceptable choice for the treatment of hypotension. If multiple doses are required, phenylephrine is the preferred drug. If the maternal heart rate is low, as may occur with spinal anesthesia with a T1–T2 sensory level, ephedrine is the better choice as phenylephrine may slow the maternal heart rate further. Rather than the choice of drug, the most important factor is the rapid correction of hypotension because uteroplacental perfusion is proportionate to blood pressure. However, this has recently been questioned. In one study of 919 mothers who underwent CD, over 30% of patients who experienced a ≤30% decrease in maternal mean arterial pressure for greater than 5 minutes had no adverse effect on the neonate.31
g. In another quantitative, systematic review of studies comparing phenylephrine and ephedrine was conducted, 7 randomized controlled trials were identified for a total of 292 patients. There was no difference between phenylephrine and ephedrine in the ability to correct maternal hypotension, but a higher incidence of maternal bradycardia occurred if phenylephrine was used. In regard to the neonate, there was no difference in the incidence of true fetal acidosis, but neonates whose mothers received phenylephrine had higher umbilical arterial pH values.32
h. Most recently, one study has suggested that norepinephrine may be useful in this setting.33
CLINICAL PEARL Either phenylephrine or ephedrine is an acceptable choice for the treatment of hypotension. If multiple doses are required, phenylephrine is the preferred drug.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








