Anesthesia for Ophthalmologic Surgery
Kathryn E. Mcgoldrick
Steven I. Gayer
Key Points
Related Matter
Open Eye Injury
Table 48-1. Requirements of Ophthalmic Surgery | |
|---|---|
|
Ocular Anatomy
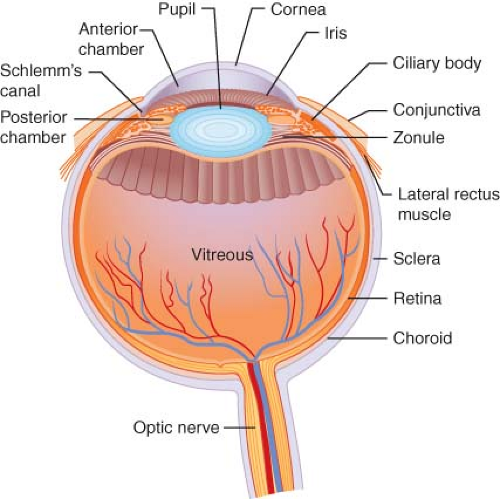
The anesthesiologist should be knowledgeable about ocular anatomy to enhance his or her understanding of surgical procedures and to aid the surgeon in the performance of regional blocks when needed4 (Fig. 48-1). Salient subdivisions of ocular anatomy include the orbit, the eye itself, the extraocular muscles, the eyelids, and the lacrimal system.
The orbit is a bony box, or pyramidal cavity, housing the eyeball and its associated structures in the skull. The walls of the orbit are composed of the following bones: Frontal, zygomatic, greater wing of the sphenoid, maxilla, palatine, lacrimal, and ethmoid. A familiarity with the surface relationships of the orbital rim is mandatory for the skilled performance of regional blocks.
The optic foramen, located at the orbital apex, transmits the optic nerve and the ophthalmic artery, as well as the sympathetic nerves from the carotid plexus. The superior orbital fissure transmits the superior and inferior branches of the oculomotor nerve; the lacrimal, frontal, and nasociliary branches of the trigeminal nerve; the trochlear and abducens nerves; and the superior and inferior ophthalmic veins. The inferior orbital or sphenomaxillary fissure contains the infraorbital and zygomatic nerves and communication between the inferior ophthalmic vein and the pterygoid plexus. The infraorbital foramen, located about 4 mm below the orbital rim in the maxilla, transmits the infraorbital nerve, artery, and vein. The lacrimal fossa contains the lacrimal gland in the superior temporal orbit. The supraorbital notch, located at the junction of the medial one-third and temporal two-thirds of the superior orbital rim, transmits the supraorbital nerve, artery, and vein. The supraorbital notch, the infraorbital foramen, and the lacrimal fossa are clinically palpable and function as major landmarks for administration of regional anesthesia.
The eye itself is actually one large sphere with part of a smaller sphere incorporated in the anterior surface, constituting a structure with two different radii of curvature. The coat of the eye
is composed of three layers: Sclera, uveal tract, and retina. The fibrous outer layer, or sclera, is protective, providing sufficient rigidity to maintain the shape of the eye. The anterior portion of the sclera, the cornea, is transparent, permitting light to pass into the internal ocular structures. The double-spherical shape of the eye exists because the corneal arc of curvature is steeper than the scleral arc of curvature. The focusing of rays of light to form a retinal image commences at the cornea.
is composed of three layers: Sclera, uveal tract, and retina. The fibrous outer layer, or sclera, is protective, providing sufficient rigidity to maintain the shape of the eye. The anterior portion of the sclera, the cornea, is transparent, permitting light to pass into the internal ocular structures. The double-spherical shape of the eye exists because the corneal arc of curvature is steeper than the scleral arc of curvature. The focusing of rays of light to form a retinal image commences at the cornea.
The uveal tract, or middle layer of the globe, is vascular and in direct apposition to the sclera. A potential space, known as the suprachoroidal space, separates the sclera from the uveal tract. This potential space, however, may become filled with blood during an expulsive or suprachoroidal hemorrhage, often associated with surgical disaster. The iris, ciliary body, and choroid compose the uveal tract. The iris includes the pupil, which controls the amount of light entering the eye by contractions of three sets of muscles. The iris dilator is sympathetically innervated; the iris sphincter and the ciliary muscle have parasympathetic innervation. Posterior to the iris lays the ciliary body, which produces aqueous humor (see “Formation and Drainage of Aqueous Humor”). The ciliary muscles, situated in the ciliary body, adjust the shape of the lens to accommodate focusing at various distances. Large vessels and a network of small vessels and capillaries known as the choriocapillaris constitute the choroid, which supplies nutrition to the outer part of the retina.
The retina is a neurosensory membrane composed of ten layers that convert light impulses into neural impulses. These neural impulses are then carried through the optic nerve to the brain. Located in the center of the globe is the vitreous cavity, filled with a gelatinous substance known as vitreous humor. This material is adherent to the most anterior 3 mm of the retina, as well as to large blood vessels and the optic nerve. The vitreous humor may pull on the retina, causing retinal tears and retinal detachment.
The crystalline lens, located posterior to the pupil, refracts rays of light passing through the cornea and pupil to focus images on the retina. The ciliary muscle, whose contractile state causes tautness or relaxation of the lens zonules, regulates the thickness of the lens.
In addition, six extraocular muscles move the eye within the orbit to various positions. The bilobed lacrimal gland provides most of the tear film, which serves to maintain a moist anterior surface on the globe. The lacrimal drainage system—composed of the puncta, canaliculi, lacrimal sac, and lacrimal duct—drains into the nose below the inferior turbinate. Blockage of this system occurs frequently, necessitating procedures ranging from lacrimal duct probing to dacryocystorhinostomy, which involves anastomosis of the lacrimal sac to the nasal mucosa.
Covering the surface of the globe and lining the eyelids is a mucous membrane called the conjunctiva. Because drugs are absorbed across the membrane, it is a popular site for administration of ophthalmic drugs.
The eyelids consist of four layers: (1) the conjunctiva, (2) the cartilaginous tarsal plate, (3) a muscle layer composed mainly of the orbicularis and the levator palpebrae, and (4) the skin. The eyelids protect the eye from foreign objects; through blinking, the tear film produced by the lacrimal gland is spread across the surface of the eye, keeping the cornea moist.
Blood supply to the eye and orbit is by means of branches of both the internal and external carotid arteries. Venous drainage of the orbit is accomplished through the multiple anastomoses of the superior and inferior ophthalmic veins. Venous drainage of the eye is achieved mainly through the central retinal vein. All these veins empty directly into the cavernous sinus.
The sensory and motor innervations of the eye and its adnexa are very complex, with multiple cranial nerves supplying branches to various ocular structures. A branch of the oculomotor nerve supplies a motor root to the ciliary ganglion, which in turn supplies the sphincter of the pupil and the ciliary muscle. The trochlear nerve supplies the superior oblique muscle. The abducens nerve supplies the lateral rectus muscle. The trigeminal nerve constitutes the most complex ocular and adnexal innervation. In addition, the zygomatic branch of the facial nerve eventually divides into an upper branch, supplying the frontalis and the upper lid orbicularis, whereas the lower branch supplies the orbicularis of the lower lid.
Ocular Physiology
Despite its relatively diminutive size, the eye is a complex organ, concerned with many intricate physiologic processes. The formation and drainage of aqueous humor and their influence on IOP in both normal and glaucomatous eyes are among the most important functions, especially from the anesthesiologist’s perspective. An appreciation of the effects of various anesthetic manipulations on IOP requires an understanding of the fundamental principles of ocular physiology.
Formation and Drainage of Aqueous Humor
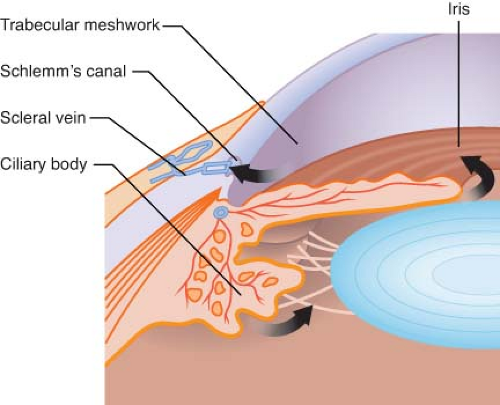
Two-thirds of the aqueous humor is formed in the posterior chamber by the ciliary body in an active secretory process involving both the carbonic anhydrase and the cytochrome oxidase systems (Fig. 48-2). The remaining third is formed by passive filtration of aqueous humor from the vessels on the anterior surface of the iris.
At the ciliary epithelium, sodium is actively transported into the aqueous humor in the posterior chamber. Bicarbonate and chloride ions passively follow the sodium ions. This active mechanism results in the osmotic pressure of the aqueous humor being many times greater than that of plasma. It is this disparity in osmotic pressure that leads to an average rate of aqueous humor production of 2 μL/min.
Aqueous humor flows from the posterior chamber through the pupillary aperture and into the anterior chamber, where it mixes with the aqueous formed by the iris. During its journey into the anterior chamber, the aqueous humor bathes the avascular lens and, once in the anterior chamber, it also bathes the corneal endothelium. Then the aqueous humor flows into the peripheral segment of the anterior chamber and exits the eye through the trabecular network, Schlemm’s canal, and episcleral venous system. A network of connecting venous channels eventually leads to the superior vena cava and the right atrium. Thus, obstruction of venous return at any point from the eye to the right side of the heart impedes aqueous drainage, elevating IOP accordingly.
Maintenance of Intraocular Pressure
IOP normally varies between 10 and 21.7 mm Hg and is considered abnormal above 22 mm Hg. This level varies 1 to 2 mm Hg with each cardiac contraction. Also, a diurnal variation of 2 to 5 mm Hg is observed, with a higher value noted on awakening. This higher awakening pressure has been ascribed to vascular congestion, pressure on the globe from closed lids, and mydriasis—all of which occur during sleep. If IOP is too high, it may produce opacities by interfering with normal corneal metabolism.
During anesthesia, a rise in IOP can produce permanent visual loss. If the IOP is already elevated, a further increase can trigger acute glaucoma. If penetration of the globe occurs when the IOP is excessively high, rupture of a blood vessel with subsequent hemorrhage may transpire. IOP becomes atmospheric once the eye cavity has been entered, and any sudden rise in pressure may lead to prolapse of the iris and lens, and loss of vitreous. Thus, proper control of IOP is critical.
Three main factors influence IOP: (1) External pressure on the eye by the contraction of the orbicularis oculi muscle and the tone of the extraocular muscles, venous congestion of orbital veins (as may occur with vomiting and coughing), and conditions such as orbital tumor; (2) scleral rigidity; and (3) changes in intraocular contents that are semisolid (lens, vitreous, or intraocular tumor) or fluid (blood and aqueous humor). Although these factors affect IOP, the major control of intraocular tension is exerted by the fluid content, especially the aqueous humor.
Sclerosis of the sclera, not uncommonly seen in the elderly, may be associated with decreased scleral compliance and increased IOP. Other degenerative changes of the eye linked with aging can also influence IOP, the most significant being a hardening and enlargement of the crystalline lens. When these degenerative changes occur, they may lead to anterior displacement of the lens–iris diaphragm. A resultant shallowness of the anterior chamber angle may then occur, reducing access of the trabecular meshwork to aqueous. This process is usually gradual, but, if rapid lens engorgement occurs, angle-closure glaucoma may transpire.
Changes in the nature of the vitreous that affect the amount of unbound water also influence IOP. Myopia, trauma, and aging produce liquefaction of vitreous gel and a subsequent increase in unbound water, which may lower IOP by facilitating fluid removal. However, under different circumstances, the opposite may occur; that is, the hydration of more normal vitreous may be associated with elevation of IOP. Hence, it is often prudent to produce a slightly dehydrated state in the surgical patient with glaucoma.
Intraocular blood volume, determined primarily by vessel dilation or contraction in the spongy layers of the choroid, contributes importantly to IOP. Although changes in arterial or venous pressure may secondarily affect IOP, excursions in arterial pressure have much less importance than do venous fluctuations. In chronic arterial hypertension, ocular pressure returns to normal levels after a period of adaptation brought about by compression of vessels in the choroid as a result of increased IOP. Thus, a feedback mechanism reduces the total volume of blood, keeping IOP relatively constant in patients with systemic hypertension.
However, if venous return from the eye is disturbed at any point from Schlemm’s canal to the right atrium, IOP increases substantially. Trendelenburg position, cervical collar, and even a tight necktie can produce increased intraocular blood volume and distention of orbital vessels, as well as attenuated aqueous drainage.5 Straining, vomiting, or coughing greatly increase venous pressure and raise IOP as much as 40 mm Hg or more. The deleterious implications of these activities cannot be overemphasized. Laryngoscopy and tracheal intubation may also elevate IOP, even without any visible reaction to intubation, but especially when the patient coughs. Topical anesthesia of the larynx may attenuate the systemic hypertensive response to laryngoscopy but does not reliably prevent associated increases in IOP.6 Ordinarily, the pressure elevation from such increases in blood volume or venous pressure dissipates rapidly. However, if the coughing or straining occurs during ocular surgery when the eye is open, as in penetrating keratoplasty, the result may be a disastrous expulsive hemorrhage, at worst, or a disconcerting loss of vitreous, at best.
Despite the notable role of venous pressure, scleral rigidity, and vitreous composition, maintenance of IOP is determined primarily by the rate of aqueous formation and the rate of aqueous humor outflow. The most important influence on formation of aqueous humor is the difference in osmotic pressure between aqueous humor and plasma. This fact is illustrated by the equation:
where K is the coefficient of outflow, OPaq is the osmotic pressure of aqueous humor, OPpl is the osmotic pressure of plasma, and CP is the capillary pressure. Hypertonic solutions such as mannitol are used to lower IOP because a small change in the solute concentration of plasma can markedly influence the formation of aqueous humor and hence IOP.
Fluctuations in aqueous humor outflow may also produce a dramatic alteration in IOP. The most significant factor controlling aqueous humor outflow is the diameter of Fontana spaces, as illustrated by the equation:
where A is the volume of aqueous outflow per unit of time, r is the radius of Fontana spaces, Piop is the IOP, Pv is the venous pressure, η is the viscosity, and L is the length of Fontana spaces. When the pupil dilates, Fontana spaces narrow, resistance to outflow is increased, and IOP rises. Because mydriasis is undesirable in both closed-angle glaucoma and open-angle glaucoma, miotics are applied conjunctivally in patients with glaucoma.
Glaucoma
Glaucoma is a condition characterized by elevated IOP, resulting in impairment of capillary blood flow to the optic nerve with eventual loss of optic nerve tissue and function. Two different anatomic types of glaucoma exist: Open-angle or chronic simple glaucoma and closed-angle or acute glaucoma. (Other variations of these processes occur but are not especially germane to anesthetic management.)
With open-angle glaucoma, the elevated IOP exists with an anatomically open anterior chamber angle. It is believed that sclerosis of trabecular tissue results in impaired aqueous humor filtration and drainage. Treatment consists of medication to produce miosis and trabecular stretching. Commonly used eye drops are epinephrine, timolol, dipivefrin, and betaxolol. Closed-angle glaucoma is characterized by the peripheral iris moving into direct contact with the posterior corneal surface, mechanically obstructing aqueous humor outflow. People who have a narrow angle between the iris and the posterior cornea are predisposed to this condition. In these patients, mydriasis can produce such increased thickening of the peripheral iris that corneal touch occurs and the angle is closed. Another mechanism producing acute, closed-angle glaucoma is swelling of the crystalline lens. In this case, pupillary block occurs, with the edematous lens blocking the flow of aqueous humor from the posterior to the anterior chamber. This situation can also develop if the lens is traumatically dislocated anteriorly, thus physically blocking the anterior chamber.
It was previously believed by some clinicians that patients with glaucoma should not be given atropine. However, this claim is untenable. Atropine in the dose range used clinically has no effect on IOP in either open-angle or closed-angle glaucoma. When 0.4 mg of atropine is given parenterally to a 70-kg person, approximately 0.0001 mg is absorbed by the eye.7 Garde et al.8 reported, however, that scopolamine has a greater mydriatic effect than atropine and recommended not using scopolamine in patients with known or suspected closed-angle glaucoma.
Equation 48-2, describing the volume of aqueous outflow per unit of time, clearly demonstrates that outflow is exquisitely sensitive to fluctuations in venous pressure. Because a rise in venous pressure produces an increased volume of ocular blood and decreased aqueous outflow, it is obvious that considerable elevation of IOP occurs with any maneuver that increases venous pressure. Hence, in addition to preoperative instillation of miotics, other anesthetic goals for the patient with glaucoma include perioperative avoidance of venous congestion and overhydration. Furthermore, hypotensive episodes are to be avoided because these patients are allegedly vulnerable to retinal vascular thrombosis.
Primary congenital glaucoma is classified according to age of onset, with the infantile type presenting any time after birth until 3 years of age. The juvenile type presents between the ages of 37 months and 30 years. Moreover, childhood glaucoma may also occur in conjunction with various eye diseases or developmental anomalies such as aniridia, mesodermal dysgenesis syndrome, and retinopathy of prematurity.
Successful management of infantile glaucoma critically depends on early diagnosis. Presenting symptoms include epiphora, photophobia, blepharospasm, and irritability. Ocular enlargement, termed buphthalmos, or “ox eye,” and corneal haziness secondary to edema are common. Buphthalmos is rare, however, if glaucoma develops after 3 years of age because by then the eye is much less elastic.
Because infantile glaucoma is frequently associated with obstructed aqueous humor outflow, management of it often requires surgical creation, by goniotomy or trabeculotomy, of a route for aqueous humor to flow into the canal of Schlemm. However, advanced disease may be unresponsive to even multiple goniotomies, and the more radical trabeculectomy or some other variety of filtering procedure may be necessary.
The juvenile form of glaucoma, in which the cornea and eye size are normal, is commonly associated with a family history of open-angle glaucoma and is treated similarly to primary open-angle glaucoma.
In cases of pediatric secondary glaucoma, goniotomy and filtering may be unsuccessful, whereas cyclocryotherapy may effect a reduction in IOP, pain, and corneal edema. The ciliary body is destroyed with a cryoprobe cooled to −70°C, thus dramatically decreasing aqueous formation.
It is essential to appreciate that the high IOP frequently encountered in infantile glaucoma can be reduced by >15 mm Hg when a surgical plane of general anesthesia is achieved. However, one study demonstrated minimal effect of halothane on IOP when the concentration ranged narrowly between 0.5% and 1%.9 Some clinicians maintain that ketamine is a useful drug to use for examination under anesthesia when infantile glaucoma is part of the differential diagnosis because ketamine does not appear to reduce IOP, giving a spuriously low reading. Moreover, even normal infants sporadically have pressures in the mid-20s. Hence, diagnosis is not based exclusively on the numerical pressure recorded under anesthesia. Other factors such as corneal edema and increased corneal diameter, tears in Descemet membrane, and cupping of the optic nerve are considered in making the diagnosis. If these aberrations are noted, surgical intervention may be mandatory, even in the setting of a reputedly normal IOP.
Effects of Anesthesia and Adjuvant Drugs on Intraocular Pressure
Central Nervous System Depressants
Controversy surrounds the issue of ketamine’s effect on IOP. Administered intravenously or intramuscularly, ketamine initially was believed to increase IOP significantly, as measured by indentation tonometry.11 Corssen and Hoy12 also reported a slight but statistically significant increase in IOP that appeared unrelated to changes in blood pressure or depth of anesthesia. However, nystagmus made proper positioning of the tonometer difficult and may have resulted in less-than-accurate measurements.
Conflicting results arose from a study in which 2 mg/kg of ketamine given intravenously to adults failed to have a significant effect on IOP.13 Furthermore, a pediatric study reported no increase in IOP after an intramuscular ketamine dose of 8 mg/kg. Indeed, values obtained were similar to those reported with halothane and isoflurane.14,15
Some of the confusion may arise from differences in premedication practices and from the use of different instruments to measure IOP. More recent studies have used applanation tonometry rather than indentation tonometry. However, even if future studies should confirm that ketamine has minimal or no effect on IOP, ketamine’s proclivity to cause nystagmus and blepharospasm
makes it a less-than-optimal agent for many types of ophthalmic surgery.
makes it a less-than-optimal agent for many types of ophthalmic surgery.
Ventilation and Temperature
Hyperventilation decreases IOP, whereas asphyxia, administration of carbon dioxide, and hypoventilation have been shown to elevate IOP.16
Hypothermia lowers IOP. On initial consideration, hypothermia might be expected to raise IOP because of the associated increase in viscosity of aqueous humor. However, hypothermia is linked with decreased formation of aqueous humor and with vasoconstriction; hence, the net result is a reduction in IOP.
Adjuvant Drugs
Ganglionic Blockers, Hypertonic Solutions, and Acetazolamide
Ganglionic blockers such as tetraethylammonium and pentamethonium cause a dramatic decrease in IOP. Trimethaphan also substantially lowers IOP in normal subjects, despite mydriasis.
Intravenous administration of hypertonic solutions such as dextran, urea, mannitol, and sorbitol elevates plasma osmotic pressure, thereby decreasing aqueous humor formation and reducing IOP. As effective as urea is in reducing IOP, intravenous mannitol has the advantage of fewer side effects. Mannitol’s onset, peak (30 to 45 minutes), and duration of action (5 to 6 hours) are similar to those of urea. Moreover, both drugs may produce acute intravascular volume overload. Sudden expansion of plasma volume secondary to efflux of intracellular water into the vascular compartment places a heavy workload on the kidneys and heart, often resulting in hypertension and dilution of plasma sodium. Furthermore, mannitol-associated diuresis, if protracted, may trigger hypotension in volume-depleted patients.
Intravenous administration of acetazolamide inactivates carbonic anhydrase and interferes with the sodium pump. The resultant decrease in aqueous humor formation lowers IOP. However, the action of acetazolamide is not limited to the eye, and systemic effects include loss of sodium, potassium, and water secondary to the drug’s renal tubular effects. Such electrolyte imbalances may then be linked to cardiac dysrhythmias during general anesthesia.
An advantage of acetazolamide is its relative ease of administration. Whereas large volumes of hypertonic solutions must be infused to reduce IOP, acetazolamide is easily given as a typical adult dose of 500 mg dissolved in 10 mL of sterile water. Acetazolamide may also be given orally, and topical carbonic anhydrase inhibitors are commercially available.
Neuromuscular Blocking Drugs
Neuromuscular blocking drugs have both direct and indirect actions on IOP.
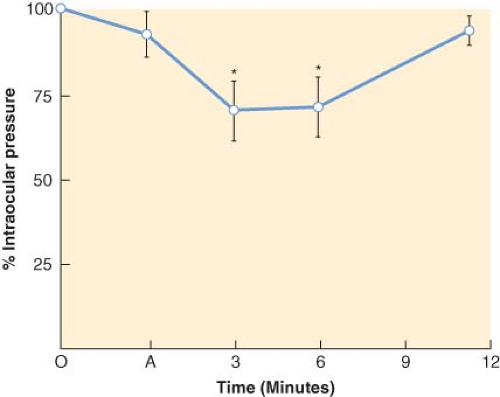
Equipotent paralyzing doses of all the nondepolarizing drugs, including pancuronium,17 directly lower IOP by relaxing the extraocular muscles (Fig. 48-3). However, if paralysis of the respiratory muscles is accompanied by alveolar hypoventilation, the latter secondary effect may supervene to increase IOP.
In contrast to nondepolarizing drugs, the depolarizing drug succinylcholine elevates IOP. Lincoff et al.18 reported extrusion of vitreous after succinylcholine administration to a patient with a surgically open eye. An average peak IOP increase of about 8 mm Hg is produced within 1 to 4 minutes of an intravenous dose. Within 7 minutes, return to baseline usually transpires.19 The ocular hypertensive effect of succinylcholine has been attributed to several mechanisms, including tonic contraction of extraocular muscles,7 choroidal vascular dilation, and relaxation of orbital smooth muscle. One study speculates that the succinylcholine-induced increase in IOP is multifactorial but primarily the result of the cycloplegic action of succinylcholine, producing a deepening of the anterior chamber and increased outflow resistance.20 Because they studied eyes with the extraocular muscles detached and still observed an elevation in IOP, these investigators proposed that changes in extraocular muscle tone do not contribute substantially to the increase in IOP observed after succinylcholine administration.
A variety of methods have been advocated to prevent succinylcholine-induced elevations in IOP. However, although some attenuation of the increase results, none of these techniques consistently and completely block the ocular hypertensive response. Prior administration of such drugs as acetazolamide, narcotics, β-blockers, and nondepolarizing neuromuscular blocking drugs has been suggested. The efficacy of pretreatment with nondepolarizing drugs is controversial.
In 1968, using indentation tonometry, Miller et al.21 reported that pretreatment with small amounts of gallamine or d-tubocurarine prevented succinylcholine-associated increases in IOP. However, in 1978, using the more sensitive applanation tonometer, Meyers et al.22 were unable to consistently circumvent the ocular hypertensive response after similar pretreatment therapy (Table 48-2). In addition, Verma23 claimed that a “self-taming” technique in which a small dose of succinylcholine is administered prior to induction was protective, but in a controlled study using applanation tonometry, Meyers et al.24 challenged this claim. Although intravenous pretreatment with lidocaine, 1 to 2 mg/kg, may blunt the hemodynamic response to laryngoscopy,6,25 such therapy does not reliably prevent the ocular hypertensive response associated with succinylcholine and intubation.26 However, Grover et al.27 claimed that pretreatment with lidocaine, 1.5 mg/kg intravenously, 1 minute before induction with thiopental and succinylcholine offered protection from IOP increases
because of succinylcholine and may therefore be of value in rapid-sequence induction for open eye injuries.
because of succinylcholine and may therefore be of value in rapid-sequence induction for open eye injuries.
Table 48-2. Effects of Succinylcholine on Intraocular Pressure: Double-Blind d-Tubocurarine or Gallamine Pretreatment | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||
The forced duction test (FDT) is an intraoperative maneuver that helps the ophthalmologist to determine whether strabismus is due to muscle paresis versus a restrictive force. It is discussed in detail in the strabismus section of this chapter. Of concern, Jampolsky28 warned that succinylcholine should be avoided in patients undergoing repeat strabismus surgery because the FDT does not return to baseline for approximately 30 minutes after administration of the drug. More recent and quantitatively sophisticated studies by Dell and Williams29 supported this caveat, although the latter investigators suggest waiting only 20 minutes after administration of succinylcholine before performing the FDT. However, in light of the black box warning issued by the Food and Drug Administration stating that use of succinylcholine in children may rarely be associated with hyperkalemia and cardiac arrest, it should be reserved for emergency intubation or when immediate airway control is needed, so the drug is typically avoided in pediatric strabismus surgery.
Oculocardiac Reflex
Reports on the alleged incidence of the oculocardiac reflex are remarkable in their striking variability. Berler30 reported an incidence of 50%, but other sources quote rates ranging from 16% to 82%. Commonly, those articles disclosing a higher incidence included children in the study population, and children tend to have more vagal tone.
A variety of maneuvers to abolish or obtund the oculocardiac reflex have been promulgated. None of these methods have been consistently effective, safe, and reliable. Inclusion of intramuscular anticholinergic drugs such as atropine or glycopyrrolate in the usual premedication regimen for oculocardiac reflex prophylaxis is ineffective.32
Atropine given intravenously within 30 minutes of surgery is believed to reduce incidence of the reflex. However, reports differ concerning dosage and timing. Moreover, some anesthesiologists claim that prior intravenous administration of atropine may yield more serious and refractory cardiac dysrhythmias than the reflex itself. Clearly, atropine may be considered a potential myocardial irritant. A variety of cardiac dysrhythmias33 and several conduction abnormalities,34 including ventricular fibrillation, ventricular tachycardia, and left bundle-branch block, have been attributed to intravenous atropine.
Although administration of retrobulbar anesthesia may provide some cardiac antidysrhythmic value by blocking the afferent limb of the reflex arc, such a regional technique is not devoid of potential complications, which include, but are not limited to, optic nerve damage, retrobulbar hemorrhage, and stimulation of the oculocardiac reflex arc by the retrobulbar block itself.
It is generally believed that the aforementioned prophylactic measures, fraught with inherent hazards, are usually not indicated in adults. If a cardiac dysrhythmia appears, initially the surgeon should be asked to cease operative manipulation. Next, the patient’s anesthetic depth and ventilatory status are evaluated. Commonly, heart rate and rhythm return to baseline within 20 seconds after institution of these measures. Moreover, Moonie et al.35 noted that, with repeated manipulation, bradycardia is less likely to recur, probably secondary to fatigue of the reflex arc at the level of the cardioinhibitory center. However, if the initial cardiac dysrhythmia is especially serious or if the reflex tenaciously recurs, atropine should be administered intravenously, but only after the surgeon stops ocular manipulation.
For pediatric strabismus surgery; however, some anesthesiologists administer intravenous atropine, 0.02 mg/kg, before commencing surgery.36 Alternatively, glycopyrrolate,
0.01 mg/kg administered intravenously, may be associated with less tachycardia than atropine in this setting.
0.01 mg/kg administered intravenously, may be associated with less tachycardia than atropine in this setting.
Anesthetic Ramifications of Ophthalmic Drugs
Anticholinesterase Agents
Echothiophate, also known as phospholine iodide, is a long-acting anticholinesterase miotic that lowers IOP by decreasing resistance to the outflow of aqueous humor. It is used to treat glaucoma that is refractory to other therapies and also to treat some children with accommodative esotropia. It is absorbed into the systemic circulation after instillation in the conjunctival sac. Any of the long-acting anticholinesterases may prolong the action of succinylcholine because, after ≥1 month of therapy, plasma pseudocholinesterase activity may be <5% of normal. It is said, moreover, that normal enzyme activity does not return until 4 to 6 weeks after discontinuation of the drug.38 Hence, the anesthesiologist should anticipate prolonged apnea after a usual dose of succinylcholine. In addition, a delay in metabolism of ester local anesthetics should be expected.
Cocaine
Cocaine, introduced to ophthalmology in 1884 by Koller, has limited topical ocular use because it can cause corneal pits and erosion. However, as the only local anesthetic that inherently produces vasoconstriction and shrinkage of mucous membranes, cocaine has been used in nasal packs during dacryocystorhinostomy. The drug is so well absorbed from mucosal surfaces that plasma concentrations are achieved that are comparable to those after direct intravenous injection. Because cocaine interferes with catecholamine uptake, it has a sympathetic nervous system potentiating effect.
The usual maximal dose of cocaine used in clinical practice is 200 mg for a 70-kg adult, or 3 mg/kg. Although 1 g is considered to be the usual lethal dose for an adult, considerable variation occurs. Furthermore, systemic reactions may appear with as little as 20 mg.
Meyers39 described two cases of cocaine toxicity during dacryocystorhinostomy, underscoring that cocaine is contraindicated in hypertensive patients or in patients receiving drugs such as tricyclic antidepressants or monoamine oxidase inhibitors. In addition, sympathomimetics, such as epinephrine or phenylephrine, should not be given with cocaine.
Obviously, before administering cocaine or another potent vasoconstrictor for dacryocystorhinostomy, the physician should carefully search out possible contraindications. To avoid toxic levels, doses of dilute solutions should be meticulously calculated and carefully administered. If serious cardiovascular effects occur, labetalol should be used to counteract them.40 Beta blocking agents should not be administered in this situation owing to the potential to exacerbate hypertension as a result of unopposed α-adrenergic stimulation. Labetalol offers the advantages of combined α-blockade and β-blockade. In addition, labetalol is preferable to esmolol because of its longer duration of action. It is important to appreciate, however, that labetalol has not been shown to reverse coronary artery vasoconstriction in humans. In the setting of cocaine-associated chest pain and/or myocardial infarction, β-blockers should not be administered acutely. Rather, nitroglycerin should be given.
Cyclopentolate
Despite the popularity of cyclopentolate as a mydriatic, it is not without side effects, which include CNS toxicity. Manifestations include dysarthria, disorientation, and frank psychotic reactions. Purportedly, CNS dysfunction is more likely to follow use of the 2% solution as opposed to the 1% solution. Furthermore, cases of convulsions in children after ocular instillation of cyclopentolate have been reported. Hence, for pediatric use, 0.5% to 1% solutions are recommended. At higher concentrations, cyclopentolate also causes cycloplegia.
Epinephrine
Although topical epinephrine has proved useful in some patients with open-angle glaucoma, the 2% solution has been associated with such systemic effects as nervousness, hypertension, angina pectoris, tachycardia, and other dysrhythmias.41 Consequently, dipivefrin hydrochloride, a prodrug of epinephrine formed by the diesterification of epinephrine and pivalic acid, is often used instead. The addition of pivaloyl groups to the epinephrine molecule enhances its lipophilic character, greatly facilitating its penetration into the anterior chamber where it reduces aqueous production and augments outflow. The prodrug delivery system is a more efficient way of delivering the therapeutic benefits of epinephrine, with less drug and with fewer side effects than conventional epinephrine therapy. Dipivefrin 0.1% is less irritating than 1% or 2% epinephrine, and, unlike cholinergic agents used to treat glaucoma, it does not produce miosis or accommodative spasm. Dipivefrin should not be used, however, in patients with narrow angles because any dilation of the pupil may trigger an attack of angle-closure glaucoma.
Phenylephrine
Pupillary dilation and capillary decongestion are reliably produced by topical phenylephrine. Although systemic effects secondary to topical application of prudent doses are rare,42 severe hypertension, headache, tachycardia, and tremulousness have been reported.
In patients with coronary artery disease, severe myocardial ischemia, cardiac dysrhythmias, and even myocardial infarction may develop after topical 10% eye drops. Those with cerebral
aneurysms may be susceptible to cerebral hemorrhage after phenylephrine in this concentration. In general, a safe systemic level follows absorption from either the conjunctiva or the nasal mucosa after drainage by the tear ducts. However, phenylephrine should not be given in the eye after surgery has begun and venous channels are patent.
aneurysms may be susceptible to cerebral hemorrhage after phenylephrine in this concentration. In general, a safe systemic level follows absorption from either the conjunctiva or the nasal mucosa after drainage by the tear ducts. However, phenylephrine should not be given in the eye after surgery has begun and venous channels are patent.
Children are especially vulnerable to overdose and may respond in a dramatic and adverse fashion to phenylephrine drops. Hence, the use of only 2.5%, rather than 10%, phenylephrine is recommended in infants and the elderly, and the frequency of application should be strictly limited in these patient populations.
Timolol and Betaxolol
Timolol, a nonselective β-adrenergic blocking drug, historically has been a popular antiglaucoma drug. Because significant conjunctival absorption may occur, timolol should be administered with caution to patients with known obstructive airway disease, congestive heart failure, or greater than first-degree heart block. Life-threatening asthmatic crises have been reported after the administration of timolol drops to some patients with chronic, stable asthma.43 The development of severe sinus bradycardia in a patient with cardiac conduction defects (left anterior hemiblock, first-degree atrioventricular block, and incomplete right bundle branch block) has been reported after timolol.44 Moreover, timolol has been implicated in the exacerbation of myasthenia gravis45 and in the production of postoperative apnea in neonates and young infants.46
In contrast to timolol, a newer antiglaucoma drug, betaxolol, a β1-blocker, is said to be more oculospecific and have minimal systemic effects.47 However, patients receiving an oral β-blocker and betaxolol should be observed for potential additive effect on known systemic effects of β-blockade. Caution should be exercised in patients receiving catecholamine-depleting drugs. Although betaxolol has produced only minimal effects in patients with obstructive airway disease, caution should be exercised in the treatment of patients with excessive restriction of pulmonary function. Moreover, betaxolol is contraindicated in patients with sinus bradycardia, congestive heart failure, greater than first-degree heart block, cardiogenic shock, and overt myocardial failure.
Intraocular Sulfur Hexafluoride
For a patient with a retinal detachment, intraocular sulfur hexafluoride or other gases, such as certain perfluorocarbons, may be injected into the vitreous to facilitate reattachment mechanically. The recommendations that follow do not apply to open-eye procedures, during which volume and pressure changes are readily compensated for by fluid and gas leak.
Stinson and Donlon48 suggested terminating nitrous oxide 15 minutes before gas injection to prevent significant changes in the size of the intravitreous gas bubble. The patient is then given virtually 100% oxygen, or a combination of oxygen and air (admixed with a small percentage of volatile agent), for the balance of the operation without adversely affecting intravitreous gas dynamics. Furthermore, if a patient requires reoperation and general anesthesia after intravitreous gas injection, nitrous oxide should be avoided for 5 days subsequent to air injection and for 10 days after sulfur hexafluoride injection49 (Table 48-3).
Perfluoropropane and octafluorocyclobutane may also be used in vitreoretinal surgery to support the retina. Like sulfur hexafluoride, these gases are relatively insoluble and require discontinuance of nitrous oxide at least 15 minutes before injection. By varying the concentration, volume, and type of gas used, bubbles can be produced that will last up to 70 days before being completely absorbed. If the patient requires reoperation, it must be remembered that perfluoropropane lingers in the eye for a protracted period.50 A Medic-Alert bracelet might be helpful in these circumstances to warn against administration of nitrous oxide during the window of vulnerability. If nitrous oxide is administered during this interval, the bubble will rapidly expand, risking retinal and optic nerve ischemia secondary to central retinal artery occlusion.
Table 48-3. Differential Solubilities of Gases | ||||||||
|---|---|---|---|---|---|---|---|---|
|
Systemic Ophthalmic Drugs
In addition to topical and intraocular therapies, various ophthalmic drugs given systemically may result in complications of concern to the anesthesiologist. These systemic drugs include glycerol, mannitol, and acetazolamide. For example, oral glycerol may be associated with nausea, vomiting, and risk of aspiration. Hyperglycemia or glycosuria, disorientation, and seizure activity may also occur after oral glycerol.
The recommended intravenous dose of mannitol is 1.5 g/kg given over a 30- to 60-minute interval. However, serious systemic problems may result from rapid infusion of large doses of mannitol. These complications include renal failure, congestive heart failure, pulmonary congestion, electrolyte imbalance, hypotension or hypertension, myocardial ischemia, and, rarely, allergic reactions. Clearly, the patient’s renal and cardiovascular status must be thoroughly evaluated before mannitol therapy.
Acetazolamide, a carbonic anhydrase inhibitor with renal tubular effects, should be considered contraindicated in patients with marked hepatic or renal dysfunction or in those with low sodium levels or abnormal potassium values. As is well known, severe electrolyte imbalances can trigger serious cardiac dysrhythmias during general anesthesia. Furthermore, people with chronic lung disease may be vulnerable to the development of severe acidosis with long-term acetazolamide therapy. Topically active carbonic anhydrase inhibitors have been developed, are now commercially available, and appear to be relatively free of clinically important systemic effects.
Preoperative Evaluation
Establishing Rapport and Assessing Medical Condition
Preoperative preparation and evaluation of the patient begin with the establishment of rapport and communication among the anesthesiologist, the surgeon, and the patient. Most patients realize that surgery and anesthesia entail inherent risks, and they appreciate
a candid explanation of potential complications, balanced with information concerning probability or frequency of permanent adverse sequelae. Such an approach also fulfills the medicolegal responsibilities of the physician to obtain informed consent.
a candid explanation of potential complications, balanced with information concerning probability or frequency of permanent adverse sequelae. Such an approach also fulfills the medicolegal responsibilities of the physician to obtain informed consent.
A thorough history of the patient and physical examination are the foundation of safe patient care. Questionnaires, in lieu of medical evaluation, lack sensitivity to detect pertinent medical issues.51 A complete list of medications that the patient is currently taking, both systemic and topical, must be obtained so potential drug interactions can be anticipated and essential medication will be administered during the hospital stay. Naturally, a history of any allergies to medicines, foods, or tape should be documented. Clearly, knowledge of any personal or family history of adverse reactions to anesthesia is mandatory. The requisite laboratory data vary, depending on the medical history and physical status of the patient, as well as the nature of the surgical procedure. Indeed, the American Society of Anesthesiologists (ASA) task force on preoperative evaluation concluded that routine preoperative tests are commonly not useful in assessing and managing patients’ perioperative experience. In a more recent multicenter study of cataract patients, for example, Schein et al.52 demonstrated that “routine” testing does not improve patient safety or outcome. Some physicians and laypersons misinterpreted the results and conclusions of this investigation, believing that patients having cataract surgery need no preoperative evaluation. It is vital to note that all patients in this trial received regular medical care and were evaluated by a physician preoperatively. Patients whose medical status indicated a need for preoperative laboratory tests were excluded from the study. Clearly, testing should be based on the results of the history and physical examination. Because “routine” testing for the >1.5 million cataract operations in the United States is estimated to cost $150 million annually, the favorable economic impact of this “targeted” approach is obvious.
Many elderly adult candidates for ophthalmic surgery are on antiplatelet or anticoagulant therapy because of a history of coronary or vascular pathology. Such patients are at higher risk for perioperative hemorrhagic events, including retrobulbar hemorrhage, circumorbital hematoma, intravitreous bleeding, and hyphema. Traditionally, antiplatelet and anticoagulant medications were withheld for an “appropriate” length of time before eye surgery. However, this strategy may increase the risk of such adverse events as myocardial ischemia or infarction, cerebrovascular accident, and deep venous thrombosis. Several studies exploring this controversial issue suggest that cataract and other ophthalmic procedures can be safely performed under regional anesthesia without discontinuing anticoagulants,53,54 especially if the prothrombin time is approximately 1.5 times control.55 A multicenter study of almost 20,000 cataract patients older than 50 years attempted to establish the risks and benefits of continuing aspirin or warfarin therapy.56 Despite the large population studied, the rate of complications was so low that absolute differences in risk were minimal. Patients who continued therapy did not have more ocular hemorrhage; those who discontinued treatment did not have a greater incidence of medical events. A recent meta-analysis of 11 studies revealed that continuing warfarin therapy for cataract patients was associated with an increased risk of bleeding, but almost all were self-limiting and not clinically relevant. No patient had bleeding-related compromise of visual acuity.57 Nonetheless, it is critical to appreciate that these investigations focused specifically on cataract operations. Oculoplastic or retinal surgery may be another matter.
Another area of potential concern involves patients whose coronary artery disease is being managed with drug-eluting stents. Although bare-metal stents are susceptible to in-stent restenosis, drug-eluting stents are more vulnerable to stent thrombosis, a complication with a high mortality rate. Thus, patients with drug-eluting stents are typically on dual antiplatelet therapy with aspirin and clopidogrel for extended periods of time. Although prospective trials are clearly needed, a conclusion that is emerging is that the risk of thrombotic complications in patients with drug-eluting stents appears to heavily outweigh the risk of bleeding complications. Therefore, given current information, a convincing case can be made for continuing dual antiplatelet therapy in the perioperative period and for delaying elective surgery for at least 4 to 6 weeks after placement of a bare metal stent and for at least 12 months after drug-eluting stent placement.57,58
Eye surgery patients are often at the extremes of age, ranging from premature babies with retinopathy of prematurity to nonagenarians. Hence, special age-related considerations such as altered pharmacokinetics and pharmacodynamics apply. In addition, elderly patients frequently have multiple comorbidities that include thyroid dysfunction, cardiopulmonary, and renal diseases. Hypertension is encountered in the majority of geriatric patients. Those with poorly controlled blood pressure should not receive dilating eye drops, such as phenylephrine, without consulting an anesthesiologist. Systemic absorption of high concentrations (e.g., >2.5% phenylephrine) or improperly instilled mydriatics can precipitate a hypertensive crisis with potentially devastating consequences.
As our society becomes increasingly geriatric, the number of ophthalmic surgery patients presenting with implanted cardiac defibrillators (ICDs) and pacemakers grows. The theoretical possibility of eye injury from patient movement in the event of ICD discharge during surgery exists. Although there is a broad spectrum of ophthalmic surgical procedures, the majority of cases use minimal bipolar cautery. For some, such as clear-corneal cataract surgery, no cautery is used. Thus, there is low risk of electromagnetic interference precipitating device discharge. Despite millions of procedures performed each year, there have not been any case reports of ICD activation during ophthalmic surgery and none of the device manufacturers have documented such an incident.59,60 A retrospective survey of ophthalmic-anesthesia providers found that >80% did not use a magnet to reprogram or inactivate an ICD before surgery.60
Perioperative movement is a possible cause of patient eye injury and potential anesthesiologist liability. An analysis of ophthalmic monitored anesthesia care (MAC) closed claims cases that resulted in blindness or poor visual outcome found that >80% were associated with inadequate anesthesia and/or patient movement either during the block or intraoperatively.61 Cough, orthopnea, and restlessness are the most common precipitators of excessive motion. Intraoperative movement during general anesthesia may also induce dire visual consequences. Because most ophthalmic surgical procedures are elective, should an enhanced risk of perioperative movement be noted during the preoperative assessment, the prudent course may be to postpone surgery until the patient is in optimal condition to remain relatively still,62 or to perform the procedure under general anesthesia. Deliberate patient selection is requisite in order to prescribe the optimal anesthesia care plan.
The anesthesiologist must be aware of the anesthetic implications of congenital and metabolic diseases with ocular manifestations. Diabetic patients often present with ocular complications, and the anesthesiologist must be knowledgeable about the systemic disturbances of physiology that affect these patients. Indeed, the list of congenital and metabolic diseases associated with ocular pathologic effects that have important anesthetic implications is lengthy. A partial summary includes syndromes such as Crouzon, Apert, Goldenhar (oculoauriculovertebral
dysplasia), Sturge–Weber, Marfan, Lowe (oculocerebrorenal syndrome), Down (trisomy 21), Wagner–Stickler, and Riley–Day (familial dysautonomia). Other diseases in this category are homocystinuria, myotonia dystrophica, and sickle cell disease.63
dysplasia), Sturge–Weber, Marfan, Lowe (oculocerebrorenal syndrome), Down (trisomy 21), Wagner–Stickler, and Riley–Day (familial dysautonomia). Other diseases in this category are homocystinuria, myotonia dystrophica, and sickle cell disease.63