INTRODUCTION
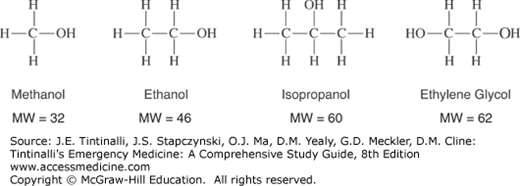
All alcohols cause clinical inebriation, with the strength of the inebriating effects directly proportional to the alcohol’s molecular weight; hence, at the same concentration, isopropanol is more intoxicating than ethanol (Figure 185-1).
Primary toxicity can be due to the parent compound (ethanol and isopropanol) or to toxic metabolites (ethylene glycol and methanol). Ethanol and isopropanol are the most common alcohols ingested; their principal effects are GI irritation and intoxication; and they do not in themselves produce metabolic acidosis. Methanol and ethylene glycol are toxic alcohols because they cause serious physiologic morbidity.
ETHANOL
Ethanol (CH3CH2OH, molecular weight 46.07) is a colorless, volatile liquid that is the most frequently used and abused drug in the world. Morbidity from acute ethanol intoxication is usually related to secondary injuries rather than direct toxic effects. Toxicity most commonly occurs from ingestion, but ethanol may also be absorbed via inhalation or percutaneous exposure.
Ethanol is readily available in many different forms. A standard alcoholic beverage, such as 12 oz (355 mL) of beer (2% to 6% ethanol by volume), 5 oz (148 mL) of wine (10% to 20% ethanol by volume), or 1.5 oz (44 mL) of 80-proof spirits (40% ethanol by volume), contains about 15 grams of ethanol. Ethanol may be found in high concentrations in many other common household products such as mouthwash (may contain up to 75% ethanol by volume), colognes and perfumes (up to 40% to 60%), and as a diluent or solvent for medications (concentration varies widely between 0.4% and 65%). Such products are often flavored or brightly colored and may be attractive to children.
Ethanol is rapidly absorbed after oral administration, and blood levels peak about 30 to 60 minutes after ingestion. The presence of food in the stomach prolongs absorption and delays the peak blood level. High concentrations of ethanol in the stomach may cause pylorospasm delaying gastric emptying. Some ethanol is broken down in the stomach by gastric alcohol dehydrogenase, which lowers the amount available for absorption. This enzyme is present at higher levels in men than in women, which may account for the fact that women usually develop a higher blood ethanol level than men after consuming the same dose per kilogram of body weight. The volume of distribution of ethanol is also gender dependent due to difference in body fat percentages: 0.6 L/kg in men and 0.7 L/kg in women.
Ethanol is a CNS depressant1 that enhances the inhibitory neurotransmitter γ-aminobutyric acid receptors and blockade of excitatory N-methyl-d-aspartic acid receptors. Modulation of these systems leads to the development of tolerance, dependence, and a withdrawal syndrome when ethanol intake ceases in dependent individuals.
Because of the phenomenon of tolerance, blood ethanol levels correlate poorly with degree of intoxication. Although death from respiratory depression may occur in nonhabituated individuals at concentrations of 400 to 500 milligrams/dL (87 to 109 mmol/L), some alcoholic individuals can appear minimally intoxicated at blood concentrations as high as 400 milligrams/dL (87 mmol/L).2 Although Canada, Mexico, and the United States have adopted 80 milligrams/dL (17 mmol/L) as the legal definition of intoxication for the purposes of driving a motor vehicle, impairment may occur with levels as low as 50 milligrams/dL (11 mmol/L), especially in nonhabituated individuals.3
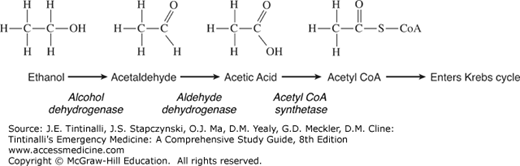
Ethanol is predominantly eliminated by hepatic metabolism, with about 10% excreted in the urine, exhaled breath, and sweat. Alcohol dehydrogenase (Figure 185-2A) is the major enzyme involved in the metabolism of ethanol, producing acetaldehyde. At low ethanol concentrations, this process follows first-order kinetics,4 but as concentrations rise, alcohol dehydrogenase becomes saturated and metabolism switches to zero-order kinetics—a fixed amount is metabolized per unit of time. Also, as ethanol concentrations rise, the hepatic microsomal oxidizing system (specifically, cytochrome P-450 2E1 [CYP2E1]) plays a more important role in metabolism.
Both alcohol dehydrogenase and CYP2E1 are inducible and thus are more active in chronic ethanol users. Therefore, rates of ethanol elimination from the blood vary from about 20 milligrams/dL per h (4 mmol/L per h) in nonhabituated individuals5 to up to 30 milligrams/dL per h (6 mmol/L per h) in individuals with chronic alcoholism.
The hallmark of ethanol toxicity is clinical inebriation.6 Behavioral disinhibition may initially appear as euphoria or agitation and combativeness. As intoxication becomes more severe, slurred speech, nystagmus, ataxia, and decreased motor coordination develop. Severe intoxication may cause respiratory depression and coma. Nausea and vomiting often occur in conjunction with neurologic depression.
Ethanol causes peripheral vasodilation and flushed, warm skin. Vasodilation causes heat loss to the environment promoting hypothermia. Vasodilation may also lead to orthostatic hypotension and reflex tachycardia. Ethanol-induced hypotension is usually mild and transient, so significant or persistent hypotension warrants investigation for alternative causes.
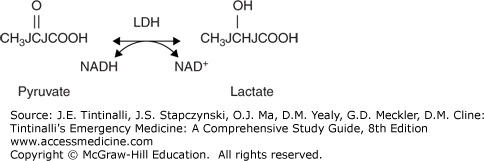
Ethanol ingestion may cause hypoglycemia, usually in children and malnourished individuals due to low glycogen stores and reduced gluconeogenesis. The metabolism of ethanol by alcohol dehydrogenase requires the presence of the oxidized form of nicotinamide adenine dinucleotide (NAD+), which is then converted into its reduced form (NADH). The metabolism of a significant amount of ethanol increases the NADH/NAD+ ratio, which then promotes the conversion of pyruvate to lactate, diverting pyruvate away from the gluconeogenesis pathway (Figure 185-2B).
When a chronic alcoholic suddenly stops consuming calories in the form of either ethanol or food, the body uses alternative fuel sources and begins to break down adipose tissue. This metabolism of fatty acids results in ketoacidosis (see chapter 226, Alcoholic Ketoacidosis).
Ethanol-intoxicated patients often have other disease processes, such as infections and traumatic injuries, so perform a detailed physical examination, looking especially for evidence of trauma, and obtain as much history as possible. Uncomplicated ethanol intoxication improves over a few hours. If depressed mental status fails to improve or deteriorates, consider other causes of altered mental status and evaluate aggressively.7,8
The clinical assessment guides the selection of laboratory tests. For altered levels of consciousness, obtain a point-of-care glucose level. Ethanol levels are not necessarily required in cases of mild or moderate intoxication when no other abnormality is suspected, but measure serum alcohol levels in patients with altered mental status of unclear cause. Clinical judgment of ethanol intoxication is unreliable, and self-reported drinking is also unreliable, particularly around levels of 100 milligrams/dL (22 mmol/L)9,10 or in alcohol-tolerant patients.11 In isolated ethanol intoxication, the presence of horizontal gaze nystagmus has a sensitivity of 70% to 80% for a blood ethanol level of 80 milligrams/dL (17 mmol/L) and a sensitivity of 80% to 90% for blood ethanol levels >100 milligrams/dL (22 mmol/L).12,13,14
Ask about concomitant drug use, especially cocaine. The attraction of abusing these drugs together may relate to the formation of the cocaine metabolite cocaethylene that, although less potent than the parent compound, has a half-life that is three to five times longer.15 The risk of sudden death among users of both drugs simultaneously is higher than that among cocaine users alone.
Ethanol ingestion is the most common cause of an osmolar gap on serum electrolyte analysis (see chapter 17, Fluids and Electrolytes) and may be associated with a mild metabolic acidosis,16 but a significant anion gap metabolic acidosis suggests the presence of lactic acidosis, ketoacidosis, or methanol or ethylene glycol toxicity.
Management is observation until sobriety.6 Activated charcoal is not useful since ethanol is rapidly absorbed; consider activated charcoal only if toxic adsorbable substances have been coingested.
Treat hypoglycemia with IV glucose 0.5 to 1 grams/kg. Although acute Wernicke’s encephalopathy can be precipitated by prolonged sustained administration of IV carbohydrate, there is no evidence that a single dose of IV glucose can cause this syndrome.17,18 The prevalence of vitamin deficiencies in acutely intoxicated ED patients is low and does not justify the routine use of IV vitamin-containing fluids.19 However, long-term drinkers are sometimes treated with IV fluids containing magnesium, folate, thiamine, and multivitamins, termed a banana bag because of the yellow color imparted by the multivitamin mixture. Wernicke’s encephalopathy is characterized by abnormal mental status, ataxia, and nystagmus, and requires daily treatment with thiamine, 100 milligrams, until normal diet is resumed. Fluid administration does not hasten alcohol elimination, so establishment of IV access for fluid administration alone is unnecessary in uncomplicated mild to moderate intoxication.20,21
Metadoxine, which is not currently available in the United States but is available in Latin America, Mexico, Asia, Africa, and Eastern Europe, enhances the metabolism of ethanol and accelerates recovery.6,22 Metadoxine is an ion pair between pyrrolidone carboxylate and pyridoxine. A dose of 900 milligrams IV is reported to double the rate at which ethanol blood levels decrease with time compared with the patient’s own metabolism.23,24
Patients with acute ethanol intoxication as the only clinical problem require ED observation until sober. Prior to discharge, reassess for an underlying mental health disorder, such as suicidal or homicidal ideation, that requires further care or hospital admission. Clinical judgment, rather than a serum ethanol level, determines the appropriateness of discharge. Discharge the patient in the care of a responsible companion. Patients treated for alcohol intoxication should not be responsible for their own transportation home.
ISOPROPANOL
Isopropanol (CH3CHOCH3, molecular weight 60.10), also known as isopropyl alcohol and 2-propanol, is a colorless, volatile liquid with a bitter, burning taste and an aromatic odor. It is found in many common, inexpensive household products, such as rubbing alcohol (usually 70% isopropanol). Isopropanol is widely used in industry as a solvent and disinfectant and is a component of a variety of skin and hair products, jewelry cleaners, detergents, paint thinners, and deicers.
Poisoning usually results from ingestion25 but may also occur after inhalation or dermal exposure in poorly ventilated areas or during alcohol sponge bathing. Isopropanol is approximately twice as potent as ethanol in causing CNS depression, and its duration of action is two to four times that of ethanol. As a result, it is often used as a substitute intoxicant by alcoholics as well as in suicide attempts.
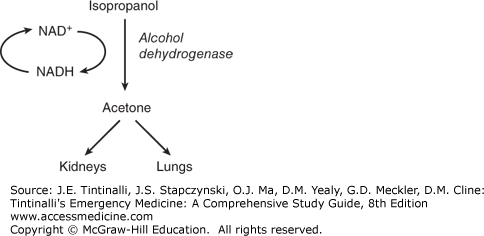
Isopropanol is rapidly absorbed from the GI tract. Its peak blood levels occur 30 to 120 minutes after ingestion, and its volume of distribution is similar to that of ethanol. The major pathway for the metabolism of isopropanol is in the liver by alcohol dehydrogenase (50% to 80%), with the remainder excreted unchanged in the urine. Isopropanol is metabolized to a ketone, not an acid (Figure 185-3).
Ketosis and an osmolar gap without acidosis are the hallmarks of isopropanol toxicity.25,26 The principal metabolite, acetone, does not cause eye, kidney, cardiac, or metabolic toxicity, although high levels of acetone may contribute to CNS depression. Acetone is excreted primarily by the kidneys, with some excretion through the lungs. It takes about 30 to 60 minutes after isopropanol ingestion for acetone to appear in the serum and about 3 hours for it to be detecTable in the urine.27
Isopropanol metabolism most closely follows concentration-dependent (first-order) kinetics. The elimination half-life of isopropanol in the absence of ethanol is 6 to 7 hours, whereas the elimination half-life of acetone is 17 to 27 hours.28 The long half-life of acetone may contribute to the prolonged mental status depression often associated with isopropanol poisoning. The toxic dose of 70% isopropanol is approximately 1 mL/kg, although as little as 0.5 mL/kg may cause symptoms. The minimum lethal dose for an adult has been reported as approximately 2 to 4 mL/kg, but survival has been reported following ingestions of up to 1 L. Children are especially susceptible to toxic effects and may develop symptoms after as little as three swallows of 70% isopropanol.29
The primary clinical toxicities of isopropanol are CNS depression caused by both the parent compound and acetone, and gastric irritation from isopropanol itself.25,26 Onset of symptoms occurs within 30 to 60 minutes after ingestion, with peak effects in a few hours and duration of symptoms for many hours longer, possibly due to the contribution of acetone. As with the metabolism of any alcohol by alcohol dehydrogenase, the increased NADH/NAD+ ratio may produce hypoglycemia.
Gastric irritation appears early and is a striking feature of isopropanol ingestions. GI symptoms range from nausea, vomiting, abdominal pain, and acute pancreatitis to hemorrhagic gastritis and upper GI bleeding. Severe poisoning is marked by early onset of coma, respiratory depression, and hypotension; rhabdomyolysis and renal failure have also been reported.
Massive ingestion may cause hypotension secondary to peripheral vasodilation. In infants and small children, if isopropanol is used to clean the skin, chemical burns can result and systemic symptoms can occur from dermal absorption.
Obtain point-of-care glucose testing and other testing as directed by the history and physical examination. Assess for upper and lower GI bleeding. Suspect isopropanol poisoning if the fruity odor of acetone or the smell of rubbing alcohol is present on the breath. Other signs are elevated osmolar gap, ketonuria, and ketonemia without acidosis. An increased anion gap or metabolic acidosis is not due to isopropanol intoxication, so if either is present, investigate for another cause.
A spurious increase in serum creatinine level as a result of acetone’s interference with the colorimetric creatinine assay is sometimes seen.30,31
Serum isopropanol and acetone levels may be assessed, although isopropanol levels may not be readily available from hospital laboratories. Isopropanol levels of 50 milligrams/dL (8 mmol/L) are often associated with intoxication in individuals who are not habituated to ethanol, but alcoholic patients may be considerably more resistant to the CNS effects of isopropanol.
Treatment is supportive. Do not administer activated charcoal or perform gastric lavage unless indicated by coingestion of an additional toxic substance. Do not administer fomepizole or ethanol, because the metabolite (acetone) is no more toxic than isopropanol itself, and preventing isopropanol metabolism may prolong CNS toxicity.
Monitor for respiratory depression, and intubate and ventilate as needed. Hypotension usually responds to IV fluids. Obtain blood for type and cross-match if needed to treat GI bleeding.
Consider hemodialysis if hypotension is refractory to conventional therapy or when the isopropanol level is >400 milligrams/dL (>66 mmol/L).25 However, there is no consensus regarding the need for hemodialysis even in life-threatening situations.31 Hemodialysis eliminates both isopropanol and acetone.
Patients with lethargy or prolonged CNS depression should be admitted to the hospital. Those who remain asymptomatic for 4 to 6 hours after ingestion may be discharged with referral for substance abuse counseling or mental health evaluation as indicated.
METHANOL AND ETHYLENE GLYCOL
Methanol and ethylene glycol are similar in that the parent compounds possess minor toxicity (both causing inebriation and some direct gastric irritation) but the major toxicity occurs when the liver metabolizes these compounds to substances that cause metabolic acidosis and end-organ damage.25,32,33,34 Treatment is primarily directed toward halting the formation of these toxic metabolites. Table 185-1 provides a summary comparison of methanol and ethylene glycol metabolism, clinical features, and treatment.
| Methanol | Ethylene Glycol | |
|---|---|---|
| Sources | Windshield cleaners; gas line antifreeze, solvents, solid fuel for stoves, adulterated alcoholic beverages, moonshine | Glycerin substitute, hydraulic fluid, antifreeze, adulterated alcoholic beverages, adulterated toothpaste (diethylene glycol) |
| Absorption | 30–60 min | 1–4 h |
| Metabolism (untreated) | Decreases 8.5 milligrams/dL per h (2.7 mmol/L per h) | Elimination half-life ≈ 3–8 h |
| Minimum lethal dose | 1 gram/kg or about 100 mL in an adult | 1.1 to 1.7 grams/kg or about 100 mL in an adult |
| Metabolism | Methanol → formaldehyde → formic acid | Ethylene glycol → glycoaldehyde → glycolic acid → glyoxylic acid → oxalic acid |
| Toxic effects | Formic acid blocks oxidative phosphorylation; metabolic acidosis from formic and lactic acids | Metabolic acidosis and tissue toxicity from glycolic acid and calcium oxalate tissue damage |
| Clinical features | Inebriation from parent compound, then 12–14 h later: metabolic acidosis; blurred or snow field vision; nausea, vomiting, abdominal pain | Inebriation from parent compound, then 4–12 h: CNS effects, hypocalcemia, metabolic acidosis; 12–24 h: multisystem organ failure; 24–72 h: renal failure |
| Diagnosis | Methanol level >20 milligrams/dL (>6 mmol/L) Early: unexplained osmolal gap >10 mOsm/kg H2O Later: elevated anion gap metabolic acidosis | Ethylene glycol level >20 milligrams/dL (>3.2 mmol/L) Early: unexplained osmolal gap >10 mOsm/kg H2O Later: elevated anion gap metabolic acidosis and calcium oxalate crystals in urine |
| Treatment | Fomepizole 15 milligrams/kg IV over 30 min and then 10 milligrams/kg IV over 30 min every 12 h OR Ethanol 10 mL/kg of 10% IV ethanol @ 100 milligrams/kg per h to keep ethanol level >150 milligrams/dL Folic acid 1 milligram/kg IV every 4–6 h (up to 50 milligrams per dose), continue until toxicity resolved IV sodium bicarbonate to maintain serum pH >7.30 See text for indications for hemodialysis | Fomepizole 15 milligrams/kg IV over 30 min and then 10 milligrams/kg IV over 30 min every 12 h OR Ethanol 10 mL/kg of 10% IV ethanol @ 100 milligrams/kg per h to keep ethanol level >150 milligrams/dL Pyridoxine 50–100 milligrams IV every 6 h for 24–48 h Thiamine 100 milligrams IV every 6 h for 24–48 h Magnesium sulfate 2 grams IV (once) IV sodium bicarbonate to maintain serum pH >7.20 See text for indications for hemodialysis |
Methanol, the simplest alcohol (CH3OH, molecular weight 32.05), is a colorless, volatile liquid with a distinctive “alcohol” odor. Methanol is used in the synthesis of other chemicals and may be found in automotive windshield cleaning solution, solid fuel for stoves and chafing dishes, model airplane fuel, carburetor cleaner, gas line antifreeze, photocopying fluid, and solvents. Trivial amounts are found in fruits and vegetables, aspartame-containing products, and fermented spirits.
Most cases of methanol poisoning occur by ingestion, and most contemporary exposures in the United States occur from unintentional ingestion of windshield washer fluid and other automotive cleaning products.35,36 Worldwide, there are outbreaks of poisoning from contaminated alcoholic beverages.37,38 Persons who wish to consume ethanol but have no access to it for financial or other reasons may consume methanol as an alternative, either intentionally or unintentionally (due to improper or confusing labeling containing the word alcohol). Methanol may be systemically absorbed after inhalation or dermal exposure, but this rarely causes significant clinical toxicity. Hence, extensive evaluation or observation is not required after minor skin or inhalational exposures.
Ethylene glycol [CH2CH2(OH)2, molecular weight 62.07] is a colorless, odorless, sweet-tasting liquid. It was considered nontoxic in the early 1900s until the first case of toxicity was reported in 1930.39 Ethylene glycol has many contemporary uses as a glycerin substitute, preservative, component of hydraulic brake fluid, foam stabilizer, component for chemical synthesis, and most commonly an automotive coolant (antifreeze).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree