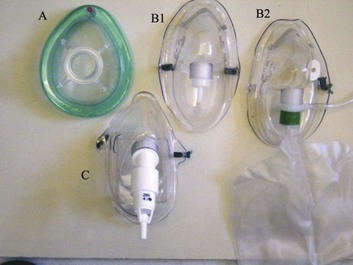
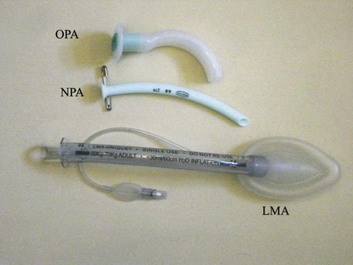
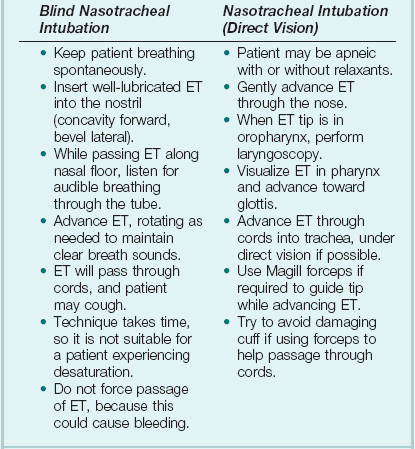
2 STRUCTURE AND FUNCTION OF THE NORMAL AIRWAY ASSESSING ADEQUACY OF THE AIRWAY PHYSIOLOGIC SEQUELAE AND COMPLICATIONS OF TRACHEAL INTUBATION CONFIRMING TUBE POSITION IN THE TRACHEA EXTUBATION IN THE DIFFICULT AIRWAY PATIENT (DECANNULATION) TUBE DISPLACEMENT IN THE CRITICAL CARE UNIT Critical care staff members require an understanding of structure and function in order to successfully manage the airway and the conditions that may affect it. The relevant information can be gained from a variety of sources.1–5 The airway begins at the nose and oral cavity and continues as the pharynx and larynx, which lead to the trachea (beginning at the lower edge of the cricoid cartilage) and then the bronchial tree. The airway1 provides a pathway for airflow between the atmosphere and the lungs;2 facilitates filtering, humidification, and heating of ambient air before it reaches the lower airway;3 prevents nongaseous material from entering the lower airway;6 and allows phonation by controlling the flow of air through the larynx and oropharynx.4 The bronchial tree is similar in structure to the trachea. Two main bronchi diverge from the carina. The right main bronchus is shorter, wider, and more vertical and runs close to the pulmonary artery and the azygos vein. The left main bronchus passes under the arch of the aorta, anterior to the esophagus, thoracic duct, and descending aorta.7 In the nose, inspired gas is filtered, humidified, and warmed before entering the lungs. Resistance to gas flow through the nose is twice that of the mouth, explaining the need to mouth-breathe during exercise when gas flows are high. Warming and humidification continue in the pharynx and tracheobronchial tree. Between the trachea and the alveolar sacs, airways divide 23 times. This network increases the cross-sectional area for the gas exchange process but also reduces the velocity of gas flow. Hairs on the nasal mucosa filter inspired air, trapping particles greater than 10 µm in diameter. Many particles settle on the nasal epithelium. Particles 2 to 10 µm in diameter fall on the mucus-covered bronchial walls (as airflow slows), initiating reflex bronchoconstriction and coughing. Ciliated columnar epithelium lines the respiratory tract from the nose to the respiratory bronchioles (except at the vocal cords). The cilia beat at a frequency of 1000 to 1500 cycles per minute, enabling them to move particles away from the lungs at a rate of 16 mm per minute. Particles less than 2 µm in diameter may reach the alveoli, where they are ingested by macrophages. If ciliary motility is defective as a result of smoking or an inherited disorder (e.g., Kartagener’s syndrome or another ciliary dysmotility syndrome), the “mucus escalator” does not work, so more particles are allowed to reach the alveoli, thereby predisposing the patient to chronic pulmonary inflammation.8 The larynx prevents food and other foreign bodies from entering the trachea. Reflex closure of the glottic inlet occurs during swallowing6 and periods of increased intrathoracic (e.g., coughing, sneezing) or intra-abdominal (e.g., vomiting, micturition) pressure. In unconscious patients, these reflexes are lost, so glottic closure may not occur, increasing the risk of pulmonary aspiration. Adequacy of the airway should be considered in four aspects: • Patency. Partial or complete obstruction will compromise ventilation of the lungs and likewise gas exchange. • Protective reflexes. These reflexes help maintain patency and prevent aspiration of material into the lower airways. • Inspired oxygen concentration. Gas entering the pulmonary alveoli must have an appropriate oxygen concentration. • Respiratory drive. A patent, secure airway is of little benefit without the movement of gas between the atmosphere and the pulmonary alveoli effected through the processes of inspiration and expiration. Upper airway obstruction has a characteristic presentation in the spontaneously breathing patient: noisy inspiration (stridor), poor expired airflow, intercostal retraction, increased respiratory distress, and paradoxical rocking movements of the thorax and abdomen.9 These resolve quickly if the obstruction is removed. In total airway obstruction, sounds of breathing are absent entirely, owing to complete lack of airflow through the larynx. Airway obstruction may occur in patients with an endotracheal tube (ET) or tracheostomy tube in situ due to mucous plugging or kinking of the tube or the patient’s biting down on a tube placed orally. If such patients are spontaneously breathing, they will have the same symptoms and signs just described. Patients on assisted (positive-pressure) breathing modes will have high inflation pressures, decreased tidal and minute volumes, increased end-tidal carbon dioxide levels, and decreased arterial oxygen saturation. The upper airway shares a common pathway with the upper gastrointestinal tract.6 Protective reflexes, which exist to safeguard airway patency and to prevent foreign material from entering the lower respiratory tract, involve the epiglottis, the vocal cords, and the sensory supply to the pharynx and larynx.10 Patients who can swallow normally have intact airway reflexes, and normal speech makes absence of such reflexes unlikely. Patients with a decreased level of consciousness (LOC) should be assumed to have inadequate protective reflexes. Oxygen demand is elevated by the increased work of breathing associated with respiratory distress11 and by the increased metabolic demands in critically ill or injured patients. Often, higher inspired oxygen concentrations are required to satisfy tissue oxygen demand and to prevent critical desaturations during maneuvers for managing the airway. A cuffed ET, connected to a supply of oxygen, is a sealed system in which the delivered oxygen concentration also is the inspired concentration. A patient wearing a facemask, however, inspires gas from the mask and surrounding ambient air. Because the patient will generate an initial inspiratory flow in the region of 30 to 60 L per minute, and the fresh gas flow to a mask is on the order of 5 to 15 L per minute, much of the tidal inspiration will be “room air” entrained from around the mask. The entrained room air is likely to dilute the concentration of oxygen inspired to less than 50%, even when 100% oxygen is delivered to the mask.12 This unwelcome reduction in inspired oxygen concentration can be mitigated by (1) using a mask with a reservoir bag, (2) ensuring that the mask is fitted firmly to the patient’s face, (3) using a high rate of oxygen flow to the mask (15 L per minute), and (4) supplying a higher oxygen concentration if not already using 100%. A patent, protected airway will not produce adequate oxygenation or excretion of carbon dioxide without adequate respiratory drive. Changing arterial carbon dioxide tension (PCO2), by changing H+ concentration in cerebrospinal fluid (CSF), stimulates the respiratory center, which in turn controls minute volume and therefore arterial PCO2 (negative feedback).11,13 This assumes that increased respiratory drive can produce an increase in minute ventilation (increased respiratory rate or tidal volume, or both, per breath), which may not occur if respiratory mechanics are disturbed. Brain injury and drugs such as opioids, sedatives, and alcohol are direct-acting respiratory center depressants. Ventilation can be assessed qualitatively by looking, listening, and feeling. In a spontaneously breathing patient, listening to (and feeling) air movement while looking at the extent, nature, and frequency of thoracic movement will give an impression of ventilation. These parameters may be misleading, however. Objective assessment of minute ventilation requires PCO2 measurement in arterial blood or monitoring of end-tidal carbon dioxide, which can be used as a real-time measure of the adequacy of minute ventilation.13 If respiratory drive or minute ventilation is inadequate, positive-pressure respiratory support may be required, and any underlying factors should be addressed if possible (e.g., depressant effect of sedatives or analgesics). Although oxygen can be administered via nasal cannula, this method does not ensure delivery of more than 30% to 40% oxygen (at most). Other disadvantages include lack of humidification of gases, patient discomfort with use of flow rates greater than 4 to 6 L per minute, and predisposition to nasal mucosal irritation and potential bleeding.14 Therefore, despite being more intrusive for patients, facemasks are superior for oxygen administration. The three main types of facemasks are shown in Figure 2.1: • The anesthesia-type facemask (mask A in Fig. 2.1) is a solid mask (with no vents) with a cushioned collar to provide a good seal. It is suitable for providing very high oxygen concentrations (approaching 100%) because entrainment is minimized and the anesthetic circuit normally includes a reservoir of gas. These masks become unacceptable for many awake patients within a few minutes because of the association with heat, moisture, and claustrophobia. • The simple facemask has vents that allow heat or humidity out but that also entrain room air. These masks have no seal and are relatively loose-fitting. Such masks may have a reservoir bag (approximately 500 mL) sitting inferior to the mask (B2 in Fig. 2.1), or may have no reservoir (B1 in Fig. 2.1). Using a simple facemask, without a reservoir bag, it is difficult to deliver an inspired oxygen concentration in excess of 50% even with tight application and 100% oxygen flow to the mask. Under the same conditions, a simple mask with a reservoir bag can produce an inspired oxygen concentration of about 80%. • The Venturi mask (C in Fig. 2.1) has vents that entrain a known proportion of ambient air when a set flow of 100% oxygen passes through a Venturi device.14 Thus, the inspired oxygen concentration (usually 24% to 35%) is known. In the absence of any concerns about cervical spine stability (e.g., with trauma, rheumatoid arthritis, or severe osteoporosis), raising the patient’s head slightly (5 to 10 cm) by means of a small pillow under the occiput can help in airway management. This adjustment extends the atlanto-occipital joint and moves the oral, pharyngeal, and laryngeal axes into better alignment, providing the best straight line to the glottis (“sniffing” position).15,16 Acute airway obstruction in the obtunded patient often due to the tongue or extraneous material—liquid (saliva, blood, gastric contents) or solid (teeth, broken dentures, food)—in the pharynx. In the supine position, secretions usually are cleared under direct vision using a laryngoscope and a rigid suction catheter.17 In some cases, a flexible suction catheter, introduced through the nose and nasopharynx, may be the best means of clearing the airway. A finger sweep of the pharynx may be used to detect and remove larger solid material in unconscious patients without an intact gag reflex. During all airway interventions, if cervical spine instability cannot be ruled out, relative movement of the cervical vertebrae must be prevented—most often by manual inline immobilization.17,18 The triple airway maneuver often is beneficial in obtunded patients if it is not contraindicated by concerns about cervical spine instability. As indicated by its name, this maneuver has three components: head tilt (neck extension), jaw thrust (pulling the mandible forward), and mouth opening.19 The operator stands behind and above the patient’s head. Then the maneuver is performed as follows: • Extend the patient’s neck with the operator’s hands on both sides of the mandible. • Elevate the mandible with the fingers of both hands, thereby lifting the base of the tongue away from the posterior pharyngeal wall. • Open the mouth by pressing caudally on the anterior mandible with the thumbs or forefingers. If the triple airway maneuver or any of its elements reduces airway obstruction, the benefit can be maintained for a prolonged period by introducing an artificial airway into the pharynx between the tongue and the posterior pharyngeal wall (Fig. 2.2). The oropharyngeal airway (OPA) is the most commonly used artificial airway. Simple to insert, it is used temporarily to help facilitate oxygenation or ventilation before tracheal intubation. The OPA should be inserted with the convex side toward the tongue and then rotated through 180 degrees. Care must be taken to avoid pushing the tongue posteriorly, thereby worsening the obstruction. The nasopharyngeal airway (NPA) has the same indications as for the OPA but significantly more contraindications20 (Box 2.1). It is better tolerated than the OPA, making it useful in semiconscious patients in whom the gag reflex is partially preserved. These artificial airways should be considered to be a temporary adjunct—to be replaced with a more secure airway if the patient fails to improve rapidly to the point at which an artificial airway no longer is needed. Such airways should not be used in association with prolonged positive-pressure ventilation. The laryngeal mask airway (LMA) is a small latex mask mounted on a hollow plastic tube.21–26 It is placed “blindly” in the lower pharynx overlying the glottis. The inflatable cuff helps wedge the mask in the hypopharynx, sitting obliquely over the laryngeal inlet. Although the LMA produces a seal that will allow ventilation with gentle positive pressure, it does not definitively protect the airway from aspiration. Indications for use of the LMA in critical care are (1) as an alternative to other artificial airways, (2) the difficult airway, particularly the “can’t intubate–can’t ventilate” scenario, and (3) as a conduit for bronchoscopy. It is possible to pass a 6.0-mm ET through a standard LMA into the trachea, but the LMA must be left in situ. The intubating LMA (ILMA), which was developed specifically to aid intubation with a tracheal tube, has a shorter steel tube with a wider bore, a tighter curve, and a distal silicone laryngeal cuff.27–30 A bar present near the laryngeal opening is designed to lift the epiglottis anteriorly. The ILMA allows the passage of a specially designed size 8.0 ET. The Combitube (esophageal-tracheal double-lumen airway) is a combined esophageal obturator and tracheal tube, usually inserted blindly.31–35 Whether the “tracheal” lumen is placed in the trachea or esophagus, the Combitube will allow ventilation of the lungs and give partial protection against aspiration. The Combitube also is a potential adjunct in the “cannot intubate–cannot ventilate” situation. Disadvantages include the inability to suction the trachea when the device is sitting in its most common position (in the esophagus). Insertion also may cause trauma, and the Combitube is contraindicated in patients with known esophageal disease or injury or intact laryngeal reflexes and in persons who have ingested caustic substances. If the foregoing interventions are not effective or are contraindicated, tracheal intubation is required. This modality will provide (1) a secure, potentially long-term airway; (2) a safe route to deliver positive-pressure ventilation if required; and (3) significant protection against pulmonary aspiration. Orotracheal intubation is the most widely used technique for clinicians practiced in direct laryngoscopy (indications and contraindications in Box 2.2). Normally, anesthesia with or without neuromuscular blockade is necessary for this procedure, which is summarized in Box 2.3. Tracheal intubation requires lack of patient awareness (as in the unconscious state or with general anesthesia) and the abolition of protective laryngeal and pharyngeal reflexes. The drugs commonly used to achieve these states are shown in Table 2.1. Anesthesia is achieved using an intravenous induction agent, although intravenous sedatives (e.g., midazolam) theoretically may be used. Opioids often are used in conjunction with induction agents because they may reduce the cardiovascular sequelae of laryngoscopy and intubation (tachycardia and hypertension) and may contribute to the patient’s unconsciousness. Table 2.1 Drugs Used to Facilitate Tracheal Intubation Nasotracheal intubation shares the problems and contraindications associated with the NPA.20 The technique usually is employed when there are relative contraindications to the oral route (e.g., anatomic abnormalities, cervical spine instability). Nasotracheal intubation may be achieved under direct vision or with use of a blind technique, either with the patient under general anesthesia or in the awake or lightly sedated patient with appropriate local anesthesia (Box 2.4). If orotracheal or nasotracheal intubation is required but cannot be achieved, then a surgical airway is required (see later). With a need for isolation of one lung from another, a double-lumen tube (having one cuffed tracheal lumen and one cuffed bronchial lumen fused longitudinally) can be used.36 The main indications are (1) to facilitate some pulmonary or thoracic surgical procedures; (2) to isolate a lung containing contaminated fluid (e.g., in lung abscess) or blood, thereby preventing contralateral spread; and (3) to enable differential or independent lung ventilation (ILV). ILV allows each lung to be treated separately—for example, to deliver positive-pressure ventilation with high positive end-expiratory pressure (PEEP) to one lung while applying low levels of continuous positive airway pressure (CPAP) only to the other. Such a strategy may be advantageous in cases of pulmonary air leak (bronchopleural fistula, bronchial tear, or severe lung trauma) or in severe unilateral lung disease requiring ventilatory support.37,38 Ventilation with a mask requires an (almost) airtight fit between mask and face. This is best achieved by firmly pressing the mask against the patient’s face using the thumb and index finger (C-grip) while pulling the mandible upward toward the mask with the other three fingers. The other hand is used to squeeze the reservoir bag, generating positive pressure. Excessive pressure from the C-grip on the mask may lead to backward movement of the mandible with subsequent airway obstruction, or a tilt of the mask with leakage of gas. If a proper seal is difficult to attain, placing a hand on each side of the mask and mandible is advised, with a second person manually compressing the reservoir bag (four-handed ventilation). Bag-valve-mask systems have a self-reinflating bag, which springs back after compression, thereby drawing gas in through a port with a one-way valve. It is important to have a large reservoir bag with a continuous flow of oxygen attached to this port in order to ensure a high inspired oxygen concentration.39,40 Bag-valve-mask ventilation usually is a short-term measure in urgent situations or is used in preparation for tracheal intubation. Ventilation of the lungs with a bag-valve-mask arrangement is difficult if required for more than a few minutes or if the patient needs to be transported. In these instances, ventilation through a sealed tube in the trachea is indicated. Orotracheal or nasotracheal intubation, surgical cricothyrotomy, and tracheostomy all achieve the same result: a cuffed tube in the trachea, allowing the use of positive-pressure ventilation and protecting the lungs from aspiration. Mechanical ventilation is discussed in Chapter 9. Apneic oxygenation is achieved using a narrow catheter that sits in the trachea and carries a flow of 100% oxygen. The catheter may be passed into the trachea via an ET or under direct vision through the larynx. This apparatus can be set up as a low-flow open system (gas flow rate of 5 to 8 L per minute) or as a high-pressure (jet ventilation) system41 and can be used to maintain oxygenation with a difficult airway either at intubation or at extubation (see later). Laryngoscopy and intubation cause an increase in circulating catecholamines and increased sympathetic nervous system activity, leading to hypertension and tachycardia. This represents an increase in myocardial work and myocardial oxygen demand, which may provoke cardiac dysrhythmias and myocardial hypoxia or ischemia. Laryngoscopy increases cerebral blood flow and intracranial pressure—particularly in patients who are hypoxic or hypercarbic at the time of intubation.42 This rise in intracranial pressure will be exaggerated if cerebral venous drainage is impeded by violent coughing, bucking, or breath-holding. Coughing and laryngospasm occur frequently in patients undergoing laryngoscopy and intubation when muscle relaxation and anesthesia are inadequate. Increased bronchial smooth muscle tone, which increases airway resistance, may occur as a reflex response to laryngoscopy or may be due to the physical presence of the ET in the trachea; in its most severe form, termed bronchospasm, this increased tone causes audible wheeze and ventilatory difficulty. Increased resistance to gas flow will occur because the cross-sectional area of the ET is less than that of the airway. This difference usually is unimportant with positive-pressure ventilation but causes a significant increase in work of breathing in spontaneously breathing patients. Resistance is directly related to 1/r4 (where r is the radius of the ET) and will be minimized by use of a large-bore ET. Gas passing through an ET, bypassing the nasal cavity, also loses the beneficial effects of warming, humidification, and the addition of traces of nitric oxide (NO).43 The effects of intubation on functional residual capacity (FRC) are complex. In patients under anesthesia, a fall in FRC is well documented. This decrease may be due to the loss of respiratory muscle tone following induction of anesthesia and the relatively unopposed effect of the elastic recoil in the lungs.43 The increased resistance to gas flow due to the presence of the ET may slow expiration, producing intrinsic PEEP (and therefore an increase in FRC) if the next inspiration begins before expiration is complete. Laryngoscopy and intubation may cause bruising, abrasion, laceration, bleeding, or displacement or dislocation of the structures in and near the airway (e.g., lips, teeth or dental prostheses, tongue, epiglottis, vocal cords, laryngeal cartilages). Dislodged structures such as teeth or dentures may be aspirated, blocking the airway more distally. Less common complications include perforation of the airway with the potential for the development of a retropharyngeal abscess or mediastinitis. Over time, erosions due to pressure and ischemia may develop on the lips or tongue (or external nares and anterior nose in patients with a nasotracheal tube) and in the larynx or upper trachea.44 These lesions result in a breach of the mucosa with the potential for secondary infection. In the case of the lips and tongue, such lesions are (temporarily) disfiguring and painful and may inhibit attempts to talk or swallow.
Airway Management in the Critically Ill Adult
Structure and Function of the Normal Airway
The Tracheobronchial Tree
Overview of Airway Function
Assessing Adequacy of the Airway
Patency
Protective Reflexes
Inspired Oxygen Concentration
Respiratory Drive
Management of the Airway
Providing an Adequate Inspired Oxygen Concentration
Establishing a Patent and Secure Airway
Airway Maneuvers
Positioning for Airway Management
Clearing the Airway
Triple Airway Maneuver
Artificial Airways
Advanced Airway Adjuncts
Tracheal Intubation
Drug
Dose (Intravenous)
Induction Agent
Propofol
1-2.5 mg/kg
Opioids
Fentanyl
1.0-1.5 µg/kg
Morphine
0.15 mg/kg
Nondepolarizing Agents
Atracurium
0.4-0.5 mg/kg
Vecuronium
0.1 mg/kg
Rocuronium
0.45-0.6 mg/kg
Depolarizing Agent
Succinylcholine (suxamethonium)
1.0-1.5 mg/kg
Providing Ventilatory Support
Bag-Valve-Mask Ventilation
Prolonged Ventilation Using a Sealed Tube in the Trachea
Apneic Oxygenation
Physiologic Sequelae and Complications of Tracheal Intubation
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Anesthesia Key
Fastest Anesthesia & Intensive Care & Emergency Medicine Insight Engine