A: Rebound hypoglycemia in a young male volunteer who received 14 ml/kg of glucose 5% over 45 min. B: Rebound hypoglycemia in a 19-year-old male who received 3.3 ml/kg of glucose 2.5% over 20 min and then half that rate over 60 min during inguinal hernia surgery. C: Plasma glucose in a 31-year-old male who received 3.3 ml/kg of glucose 2.5% over 80 min during laparoscopic cholecystectomy. D: Slow elimination of glucose in a 73-year-old male who received 3.3 ml/kg of glucose 2.5% over 20 min and then half that rate over 60 min during open colon surgery.
Bicarbonate is usually marketed in a hypertonic solution (600–800 mosmol/kg). Bicarbonate may be use to combat life-threatening acidosis. The use of bicarbonate solutions is quite limited because the rational treatment of acidosis is to seek and treat the underlying cause. The end product in the metabolism is carbon dioxide and water, which means that administration of bicarbonate increases breathing, which is an issue for patients with pulmonary disease.
Colloid fluids, general
All colloid fluids are associated with a risk of anaphylaxis. The risks, as quantified in a French multicenter study of almost 20,000 patients, were 0.35% for gelatin, 0.27% for dextran, 0.10% for albumin, and 0.06% for HES. The reactions were considered to be severe in 20% of the patients.[18] Reactions were more common in males and tripled in patients with known drug allergy. These reactions make it necessary to have drugs for acute treatment of anaphylaxis at hand when providing a colloid fluid. In many countries, a hapten inhibitor in the form of a very small dextran molecule (1 kDa) is available, which greatly reduces the number of allergic reactions to dextran and further makes those that develop very mild.
Mild allergic reactions show as a feeling of warmth in the skin, possibly together with erythema and itching. Nausea may develop. More severe reactions consist of various degrees of arterial hypotension and bronchospasm.
All colloid fluids, but particularly the hyper-oncotic preparations such as 10% HES and dextran 40, may induce pre-renal anuria in dehydrated patients.[19,20] This problem might in part be due to tissue deposition of the colloid molecules. A more simplistic explanation is that the filtration pressure in the glomeruli becomes insufficient when the colloid osmotic pressure in the plasma is artificially increased at the same time as the hydrostatic pressure is low.
Colloid fluids, specific
Large amounts of albumin place a metabolic burden on the patient, as the protein is split into amino acids that are incorporated into new proteins or used as energy. The nitrogen content can be difficult to excrete for patients with impaired kidney function. The long half-life of exogenous albumin (2 weeks) can make peripheral edema particularly long-lasting. This is a concern in diseases, such as sepsis, in which the increased capillary leakage rate might be greater than the capacity of the lymphatic system to remove the colloid. Albumin should be avoided in head injury, where normal saline is a better choice for intravenous volume support.[21]
Hydroxyethyl starch undergoes a sequential metabolism but a smaller fraction of the molecules accumulates in the reticuloendothelial system, where it can be found even after several years. Repeated infusions of starch over several days can cause long-standing (1–2 years) and troublesome itching that is probably due to tissue deposition of starch.[22] Several weeks may elapse between HES exposure and the onset of pruritus. There is some evidence that HES (Voluven) has a higher tendency than crystalloid fluid to cause nausea and vomiting after laparoscopic surgery.[23]
HES is associated with a dose-dependent increased likelihood of renal impairment and need for renal-replacement therapy. This has been shown in septic patients [24–26] and in patients subjected to general intensive care.[27] The same findings have not been made when HES is used in the operating room. During aortic repair HES is even better than gelatin in preserving the kidney function,[28] which is opposite to the findings made in septic patients.[24]
Dextran does not leave any metabolic residues in the body. The risk of severe anaphylactic reactions is a concern, and infusing this colloid without first injecting a hapten inhibitor must be questioned practice. Dextran colloid improves microcirculatory flow which gives rise to “oozing,” i.e. that cut surfaces bleed from a larger number of capillaries. Already by 1988 there were 10 randomized controlled trials available of dextran as a drug for the prevention of thromboembolism; pooling of the data showed an incidence of 15.6% among dextran-treated patients and 24.2% in the controls.[29]
Gelatin has been claimed to reduce the function of fibronectin, a plasma factor of importance to wound healing and phagocytosis.[30] The clinical importance of this finding is unclear. Well-known problems associated with gelatin mainly consist of anaphylactic reactions, which often are histamine-dependent. Most reactions present as urticaria or transient fever, but severe allergic edema may also occur. There is cross-reactivity between different preparations of gelatin.[31]
Peripheral edema
All infusion fluids may cause postoperative weight gain and peripheral edema. The mechanisms are partially known but include the following:
Colloid fluids induce peripheral edema when inflammation-induced shedding of the endothelial glycocalyx increases the capillary leak of proteins. The leakage, which also includes the macromolecules of the colloid solution, must be transported back to the plasma by the lymphatic system, which may be overwhelmed by the increased burden. In septic shock, one can reckon with a four-fold increase of the capillary leakage rate. Hence, colloid-induced peripheral edema develops because of a discrepancy between capillary leakage rate and the capacity of the lymphatic system, whereby a surplus of fluid is held in the interstitium by oncotic forces (“queue theory”) instead of being excreted.
There is experimental evidence to support that peripheral edema can also develop without an increase of the capillary escape rate. The cause is probably the vastly different half-lives for the infused colloid macromolecules than for their associated plasma volume expansion. The former half-life is much longer than the latter, showing that the macromolecules reside outside the bloodstream for a considerable time after an infusion. Although the infusion may cause an appropriate diuretic response during which the entire infused volume is excreted, even slowly leaking macromolecules increase the colloid osmotic pressure of the interstitial fluid space, which is normally only half of that measured in the plasma.[1] In the interstitium, their oncotic strength binds fluid volume from subsequently infused crystalloids. This phenomenon can be regarded as an interaction effect between a colloid and a crystalloid; as an example, infusing Ringer’s acetate almost 2 hours after Voluven was followed by a much more peripheral accumulation of fluid and a smaller urinary excretion than when Ringer’s acetate was infused alone (Figure 34.2).[32]
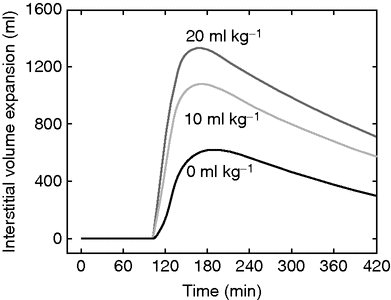
Volume expansion of the interstitial fluid space when 20 ml/kg of Ringer’s acetate is infused over 30 min in 10 male volunteers (mean body weight 79 kg) beginning at 105 min, preceded by an infusion of either no starch, or 10 or 20 ml/kg of hydroxyethyl starch 130/0.4, between 0 and 30 min. The crystalloid fluid has a preferential peripheral distribution when preceded by starch.
Crystalloid fluid is often said to cause peripheral edema because such fluid is distributed over the entire extracellular fluid space. This view is simplistic since large parts of the interstitial fluid space have very low compliance to volume expansion, some low enough to be virtually impossible to expand with isotonic crystalloid fluids. These parts include bone tissue, the brain, and organs surrounded by a tight capsule. Crystalloid fluid accumulates preferentially in interstitial areas with the highest compliance to volume expansion, such as the skin and gastrointestinal tract. Volume kinetic studies suggest that crystalloid fluid usually fills 2/3 of the expected size of the interstitial fluid space. Studies showing a 20%/80% distribution of crystalloid fluid between the plasma and the interstitium never take the urinary excretion into account. A more correct figure after a 30-min equilibration period would be 20%/50%/30% (plasma/interstitium/urine) in a volunteer and 30%/60%/10% during surgery. However, the amount that resides in the interstitium is smaller with slow infusions and higher with rapid infusions.
Edema is rarely an issue in volunteer experiments because the urinary excretion is very prompt. However, anesthesia and surgery are associated with marked fluid retention. Here, the half-life of balanced crystalloids increases from 20–50 min to several 100 min. This adds to the volume expansion of both the plasma and the interstitial fluid space, and therefore promotes edema.
A second mechanism that promotes edema from crystalloid fluid is that the fibers of the interstitial fluid matrix lose elasticity when volume-expanded, whereby fluid deposited in the interstitial fluid gel has difficulty in returning to the plasma. The interstitium behaves like a filled balloon that does not return to its original size when deflated. If the fluid that remains in the plasma is excreted, the altered elasticity of the interstitial fibers gives rise to the paradoxical situation in which peripheral edema is present alongside hypovolemia. The lost elasticity is dependent on the rate of infusion and is quite apparent after infusion of crystalloid fluid at the rate of 50 ml/min for 30 min (about 2 liters). The anesthetist who wants to limit this problem should provide a continuous infusion at a rate no faster than 10 ml/min, where actually very little of the infused fluid even enters the interstitial fluid space because of the compliance issue.[33,34] Very large volumes of crystalloid fluid even break up the interstitial fluid matrix, and the fluid forms lacunae in the interstitium, which makes it very difficult to recruit and make available for excretion.[35,36] This break-up of the interstitium is best studied in isolated lung preparations.[37,38] The situation might be expected to be worsened by the fact that albumin, which is normally excluded from parts of the interstitium, can penetrate into opened parts of the interstital gel and bind fluid by its oncotic strength.[39]
How much the complex neuroendocrine response to trauma contributes to the development of peripheral edema is difficult to quantify. The response includes a rise in plasma glucose, which recruits fluid from the intra- to the extracellular fluid space, and elevations of water-retaining hormones such as aldosterone, vasopressin, and cortisol. Beta-1-adrenergic stimulation acts to retain fluid in the extracellular fluid space. Alpha-1-adrenergic stimulation increases the rate of elimination of infused crystalloid fluid,[40,41] as does the rise in atrial natriuretic peptide concentration occurring from vigorous plasma volume expansion. None of these hormones specifically changes the distribution of fluid to the interstitium except for atrial natriuretic peptide, which, together with activation of the inflammatory cascade, increases the capillary leakage of macromolecules (e.g. proteins) from the plasma. At the same time, the atrial natriuretic peptide and probably also inflammation increase the urinary excretion, and thus the total effect on the body may consist mainly of a reduction of the plasma volume.
A special mechanism that promotes peripheral edema is found in burns. Here, there is a marked drop in the interstitial hydrostatic pressure, which more or less suctions fluid from the intravascular space to peripheral tissues.
Death from overhydration has been studied in animals. The hearts of mice receiving large amonts of isotonic saline show changes similar to those occurring from irrigating fluid, namely severe interstitial dilatation with fluid lacunae that compress the capillary lumina, making blood flow difficult.[42] Rupture and fragmentation are common features of this process (Figure 34.3).
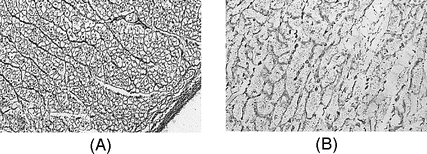
A: Normal cytoarchitecture of a pig’s heart after receiving 150 ml/kg of mannitol 3% over 90 min. Lumina of the vessels are preserved (×200; field of view 300 μm). B: Destroyed cytoarchitecture in the subendocardium in a mouse that died after receiving 300 ml/kg of isotonic saline over 60 min. The reticular fibers are fragmented and degenerated (×600; field of view 100 μm). Light microscopy with Gordon and Sweet’s silver impregnation for reticulum fibers.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




