Chapter 67
Acute Neuromuscular Weakness
General Approach to Acute Neuromuscular Weakness
Need for Ventilatory Support
In the setting of worsening respiratory weakness or clinical concern about the patient’s ability to protect their airway, the clinician should be aggressive about elective intubation. With close monitoring, endotracheal intubation should be performed as an elective procedure before precipitous respiratory collapse (i.e., not in response to this complication). As long as the patient does not need an artificial airway to clear secretions or prevent airway occlusion, noninvasive ventilation may serve as a useful temporizing modality to provide assisted ventilation, particularly if the respiratory muscle weakness can be reversed quickly (see Chapter 3), but concern for aspiration is an important consideration in this scenario.
The following parameters warn of impending respiratory arrest and, generally, are indications for intubation (Box 67.1): vital capacity (VC) less than 20 mL/kg predicted body weight (PBW) (see footnote of Box 67.1 for formulas to calculate PBW); VC falling toward 20 mL/kg PBW plus clinical signs of respiratory muscle fatigue, such as increasing respiratory rate, tachycardia, use of accessory muscles of respiration, and paradoxical motion of the diaphragm (“respiratory paradox”); maximal inspiratory pressure (MIP) worse (i.e., less negative) than –30 cm H2O; or maximal expiratory pressure < 40 cm H2O. This general guide has been termed by some the “20-30-40 rule.” Oropharyngeal weakness with an inability to handle secretions and risk of aspiration or airway obstruction also may necessitate intubation.
Differential Diagnosis
Disorders that produce neuromuscular weakness severe enough to result in ICU care, such as myasthenia gravis or the Guillain-Barré syndrome, are generally separated from disorders that develop de novo in the ICU setting (the latter are discussed in Chapter 48).
Not infrequently, the cause of the neuromuscular weakness that results in ICU care is unknown. This necessitates a diagnostic workup because pharmacologic and other interventions vary according to specific causes. In the differential diagnosis of acute neuromuscular weakness, certain clues from the history and examination can help to identify its cause (Table 67.1).
TABLE 67.1
Differential Diagnosis of Acute Neuromuscular Weakness Necessitating ICU Care§
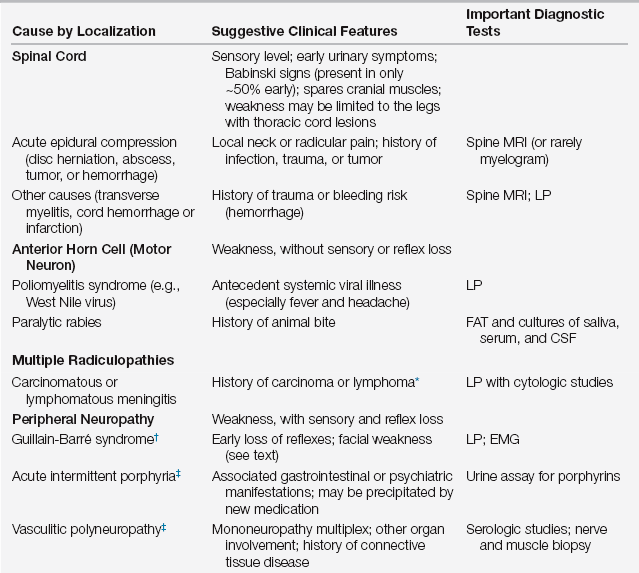
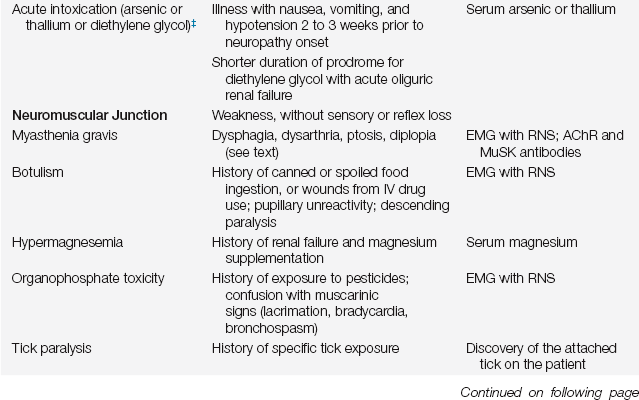

∗Neurologic disorder often the presenting feature.
§These are causes of acute neuromuscular weakness in patients who were previously well neurologically. There are many other causes of chronic neuromuscular weakness that are not listed above (e.g., ALS or muscular dystrophies) that can transiently worsen due to another illness (such as infection).
Electrophysiologic studies (nerve conduction studies and needle electromyography, which are collectively called electromyography [EMG]) are usually required to fully investigate these disorders. This is particularly true in individuals with an abnormal mental status (common in the ICU setting) who may not be able to cooperate with a motor and sensory examination. EMG allows full investigation of the presence and nature of peripheral motor and sensory involvement. It can distinguish among disorders of nerve, muscle, and neuromuscular junction. It also provides prognostic information by quantifying the extent of nerve or muscle injury.
The Guillain-Barré Syndrome
Clinical Presentation and Symptomatic Management
The clinical presentation of GBS usually consists of distal limb numbness and paresthesias, followed by progressive ascending limb weakness. The pace of progression can vary dramatically, from rapid (complete quadriparesis and intubation over 24 to 48 hours) to slow (progression over 3 or 4 weeks). In addition, respiratory decline may not always parallel the degree of limb weakness and must be followed carefully.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



