Fig. 65.1
Mesenteric angiography with coil embolization. Angiography of the colic branches with positive blush sign (Panel A and B) and following coil embolization (Panel C)
Principles of Management
Definitions
Acute lower gastrointestinal bleeding (LGIB) historically refers to gastrointestinal bleeding of recent onset (less than 3 days) from a location distal to the ligament of Treitz, located at the third portion of the duodenum, resulting in hemodynamic instability, anemia, and/or the need for blood transfusions [1]. This clinical presentation of hemodynamically unstable hematochezia (i.e., passage of fresh blood mixed with stool) differs from the majority of lower gastrointestinal bleeding episodes that tend to be self-limited bleeding and usually follows an uncomplicated clinical course [1–3]. A patient with acute LGIB may have similar clinical features to a patient with a brisk upper gastrointestinal bleed (UGIB); therefore, such a patient must be urgently evaluated for this possibility. Given that nearly all of LGIB are related to diverticular disease, ischemic colitis, vascular angiodysplasias, hemorrhoids, and colorectal cancer, it is not surprising that most patients are older (mean age at presentation ranges from 63 to 77 years) and have multiple other comorbidities, such as underlying cardiovascular, liver, and/or renal disease (Table 65.1).
Table 65.1
Differential diagnosis for lower gastrointestinal bleeding
Etiology | Frequency (%) | Comments |
|---|---|---|
Diverticulosis | 20–65 | Presents with painless hematochezia; usually resolves spontaneously in 75–80 % of patients |
Angioectasia (also known as angiodysplasias or vascular ectasias) | 3–15 | More than two-thirds seen in patients aged >70 years; risk factors include advanced age, comorbidities, presence of multiple angioectasias, and use of anticoagulants or antiplatelet drugs |
Ischemic colitis | 1–19 | Presents with sudden onset of abdominal cramping, followed by hematochezia; “watershed” areas of colon: splenic flexure and rectosigmoid junction typically affected |
Hemorrhoids | 2–64 | May be incidental finding in up to 75 % of LGIB; typically present with painless, intermittent, scant hematochezia |
Colorectal cancer | 17 | Typically present with bowel habit changes and unintentional weight loss; right-sided colonic tumors more commonly cause occult bleeding and left-sided tumors often cause hematochezia |
Post-polypectomy bleeding | 2–8 | Complication of colonoscopy |
Inflammatory bowel disease | N/A | Uncommonly present with LGIB requiring hospitalization |
Initial Evaluation
A focused history and physical examination is essential in the initial evaluation of a patient with acute LGIB. Key elements of the history include characteristics and duration of current bleeding (e.g., stool color, frequency), any associated symptoms (e.g., abdominal pain, recent change in bowel habits, fever, urgency/tenesmus, weight loss), history of similar prior bleeding episodes, relevant past medical history (e.g., recent endoscopy with polypectomy, trauma, previous abdominal surgeries, history of peptic ulcer disease or inflammatory bowel disease, etc.), and current medications (e.g., nonsteroidal anti-inflammatory drugs (NSAIDs), aspirin, anticoagulants, etc.) [1, 3, 4]. Initial and serial measurements of the patient’s vital signs are essential. Resting supine tachycardia, tachypnea, and hypotension are nonspecific, but sensitive, signs of a critically ill patient. Postural changes in blood pressure (decrease by >10 mmHg) and/or heart rate (increase by 10 beats/min) from supine to standing position (i.e., orthostasic hypotension) correlates with an acute blood loss of more than 800 mL (or about 15 % of total circulatory blood volume). Marked tachycardia, tachypnea, hypotension, and depressed mental status correlates with an acute blood loss of more than 1500 mL (or about 30 % of total circulatory blood volume) [5]. In addition to focused neurologic, cardiac, pulmonary, and abdominal examinations, a digital rectal examination should be performed. Initial laboratory assessment should include a complete blood cell count, serum electrolytes, renal indices, liver function tests, coagulation profile, type and crossmatch. Additional studies such as a chest x-ray and/or electrocardiogram should be individualized to those patients also presenting with cardiopulmonary symptoms or risk factors for development of complications.
Initial Resuscitation and Management
Hemodynamic monitoring of vital signs, rapid establishment of intravenous access, and initiation of volume resuscitation should take place without delay in the emergency department. Hemodynamic monitoring should include continuous assessment of heart rate, blood pressure, respiratory rate, and oxygen saturation by pulse oximetry. At least two large-bore diameter (18 gauge or greater) peripheral intravenous (PIV) catheters (18G or greater) should be promptly placed. Advantages of PIVs over central venous catheters (CVC) are multiple in this setting: (1) quickly establish intravenous access, (2) ease of placement by nursing staff, (3) less invasive than CVCs with reduced chance for potentially significant complications, especially in those who are hemodynamically unstable and/or coagulopathic, and (4) allowance for more rapid volume resuscitation compared to CVCs due to the shorter length and larger diameter. These advantages of PIVs must be weighed against their disadvantages that include less secure intravenous access, susceptibility to dislodgement, and inability to administer multiple medications safely, such as vasopressors. Crystalloid formulations, such as 0.9 % saline or lactated Ringer’s solution, are the fluids of choice for initial resuscitation. Decisions regarding transfusion of red blood cells and other blood products, such as fresh frozen plasma and/or platelets, should be individualized, carefully weighing potential benefits in the setting of active symptomatic bleeding with the potential risks of volume overload or transfusion reactions.
Appropriate Patient Disposition
Following initial resuscitative measures in the emergency department, reassessment of the patient with acute LGIB must be performed to determine clinical stability for specific diagnostic and/or therapeutic interventions. If there remains persistent hemodynamic instability, further resuscitation is necessary with crystalloids and/or blood products (as needed) and central venous access should be established for invasive monitoring and possible use of vasopressors. At this time, the patient should be transferred to the ICU for close hemodynamic and cardiopulmonary monitoring. Although there have been multiple risk prediction scores and models reported for acute LGIB, none have been adopted in everyday clinical practice yet (see below). In general, patients with clinical evidence of ongoing or severe bleeding, those with a transfusion requirement of greater than two units of PRBCs, and those with significant comorbidities may require admission and monitoring in an ICU [6, 7].
Nasogastric Tube Lavage
Expert guidelines generally favor an algorithmic approach for determining the etiology of acute LGIB (Fig. 65.2) [1, 2]. This most commonly occurs as the result a colonic bleeding source; however, hematochezia in the setting of hemodynamic compromise should raise the clinical suspicion for a brisk UGIB [4, 8]. Therefore, patients presenting with acute LGIB should be evaluated with a NGT lavage. If gastric lavage aspirate is positive for blood, or is clear without either blood or bile, an upper GI source of bleeding has not yet been ruled out [9] and urgent gastroenterology consultation should be requested for esophagogastroduodenoscopy (EGD). Aspiration of bilious fluid in the absence of blood on NGT lavage makes UGIB unlikely. A lower gastrointestinal source of bleeding should be suspected and appropriate diagnostic testing for LGIB should now ensue.
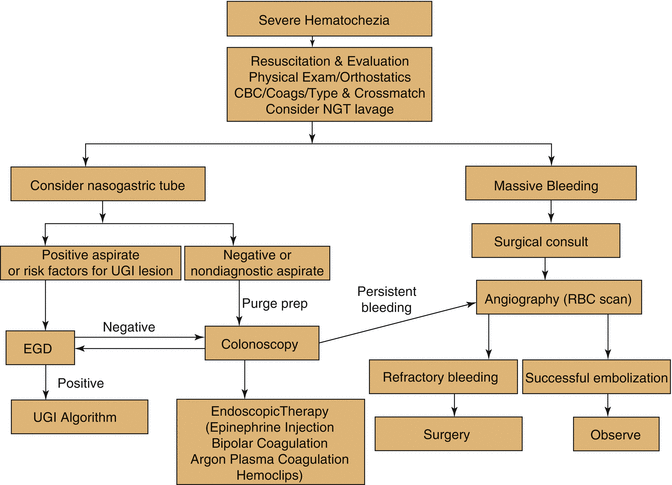

Fig. 65.2
Algorithm for management of LGIB (From ASGE Guidelines [2] with permission from Elsevier Limited)
Urgent Colonoscopy
Patients with acute LGIB who have been successfully resuscitated and are clinically stable without evidence of ongoing hemorrhage are candidates for urgent colonoscopy. This single procedure may be both diagnostic and therapeutic. According to the most recent American Society of Gastrointestinal Endoscopy (ASGE) guidelines published in 2014, urgent colonoscopy is recommended within 24 h of admission after a rapid bowel preparation in the evaluation of patients with severe hematochezia (per ASGE guidelines, this recommendation is supported by moderate quality of evidence) [2]. The overall diagnostic yield of colonoscopy in the evaluation of acute LGIB ranges from 45 to 100 %, according to multiple studies [10–12]. The most common site of bleeding in the largest series by Rossini et al. was the left colon caused most often by ulcerated carcinomas and diverticular disease [10, 13]. Colon preparation with polyethylene glycol-based solutions administered enterally (either by mouth or via NGT) is important before colonoscopy to improve visualization, increase the diagnostic yield, and reduce the risk of perforation [14]. Importantly, there is no evidence of a deleterious effect on the rate of hemorrhage with bowel preparation. Several modalities are available for endoscopic treatment of LGIB. Endoscopic treatment with epinephrine solution injection combined with thermal coagulation or endoscopic clip placement as the preferred management in patients presenting with diverticular bleeding (high quality of evidence according to ASGE guidelines) [2]. Endoscopic treatment with argon plasma coagulation as preferred management in patients with bleeding angioectasis (high quality of evidence according to ASGE guidelines) [2].
Mesenteric Angiography
For patients with severe acute LGIB with massive ongoing bleeding, hemodynamically unstable for colonoscopy, unable to be prepped, and/or for those who have failed endoscopic management, emergency consultation with interventional radiology for mesenteric angiography is essential. Mesenteric angiography can detect bleeding rate at 0.5 mL/min [15]. Superselective embolization with microcoils, polyvinyl alcohol particles, or water-insoluble gelatin (gel foam) has improved the success rate of this technique and decreased occurrence of adverse event of bowel infarction. A meta-analysis of angiography and embolization as first-line therapy for acute LGIB found embolization to be an effective treatment for diverticular bleeding, with successful hemostasis in 85 % of patients as compared to 50 % of those with bleeding from other sources at 30-day follow-up [16]. In contrast, rebleeding after embolization for non-diverticular bleeding, such as angioectasias, may occur in greater than 40 % of patients [6].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







