INTRODUCTION AND EPIDEMIOLOGY
Acute kidney injury is the deterioration of renal function over hours or days resulting in the accumulation of toxic wastes and the loss of internal homeostasis. Definitions based on renal function are listed in Table 88-1.1
| AKIN Stage | RIFLE Category | GFR Criteria | Urine Output Criteria |
|---|---|---|---|
| Stage 1 | Risk | Serum Cr increased 1.5 times* or (AKIN only) Cr increase >0.3 milligrams/dL over <48 h* or GFR decrease 25%–50%* | 0.5 mL/kg/h for 6 h |
| Stage 2 | Injury | Serum Cr increased 2.0–3.0 times* or GFR decrease 50%–75%* | 0.5 mL/kg/h for 12 h |
| Stage 3 | Failure | Serum Cr increased >3.0 times* or Cr >4 milligrams/dL and acute increase >0.5 milligrams/dL* or GFR decrease >75%* | 0.3 mL/kg/h for 24 h or Anuria for 12 h |
| N/A | Loss | Complete loss of kidney function for >4 wk | |
| End-stage renal disease | Need for renal replacement therapy for >3 months |
Community- and hospital-acquired kidney injuries differ by cause, treatment, and outcome (Table 88-2). Community-acquired renal failure is diagnosed in only 1% of hospital admissions at the time of presentation2,3 and is usually secondary to volume depletion; thus, the vast majority of cases presenting to the ED have a reversible cause.3 Mortality among patients presenting to the ED with prerenal acute renal failure may be as low as 7%.4
Hospital-acquired renal failure is only apparent after admission.5 Hospital factors include advanced patient age, potential nephrotoxic exposures in a hospital setting, sepsis,6 and multiorgan system illness in hospitalized patients. There is an almost linear relationship between increasing severity of renal injury and mortality rate: no renal injury, 4.4%; risk category/stage 1, 15.1%; injury category/stage 2, 29.2%; and failure category/stage 3, 41.1%.6
PATHOPHYSIOLOGY
Renal insult is classified as prerenal (decreased perfusion of a normal kidney), intrinsic (pathologic change within the kidney itself), or postobstructive (obstruction to urine outflow).
The functions of the kidneys are glomerular filtration, tubular reabsorption, and secretion. Normal glomerular filtration rate (GFR) in early adulthood is approximately 120 mL/min/1.73 m2 and typically decreases by 8 mL/min/1.73 m2 every decade thereafter. The driving force for glomerular filtration is glomerular capillary pressure, which depends on renal blood flow and autoregulation. For most causes of acute renal failure, global or regional decrease in renal blood flow is the final common pathway. Recovery from acute renal failure first depends on restoration of renal blood flow.
In prerenal failure, tubular and glomerular functions are still maintained. Restoration of circulating blood volume is usually sufficient to restore function. Postobstructive renal failure initially results in an increase in tubular pressure, which decreases the driving force for filtration. This pressure gradient soon equalizes, and the maintenance of depressed GFR depends on vasoconstrictors. Rapid relief of urinary obstruction in postrenal failure results in a prompt decrease of vasoconstriction.
Intrinsic renal failure occurs with diseases of the glomerulus, small vessels, interstitium, or tubule and is associated with the release of renal vasoconstrictors. The most common cause of intrinsic renal failure is ischemic injury or ischemic tubular necrosis (also historically called acute tubular necrosis), when renal perfusion is decreased so much that the kidney parenchyma is affected.
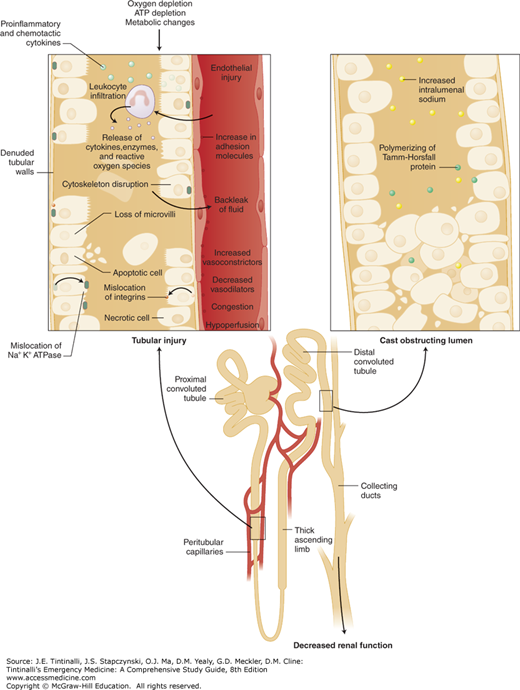
During the period of depressed renal blood flow, the kidneys are especially vulnerable to further insults. Exposure at this time to known nephrotoxins such as radiocontrast agents and aminoglycosides causes iatrogenic acute renal failure. Figure 88-1 illustrates the cellular and subcellular events leading to ischemic tubular necrosis.
In intrinsic renal failure, clearance of tubular toxins and initiation of therapy for glomerular diseases decrease vasoconstriction and help restore renal blood flow. Once the cause of injury is resolved, the remaining functional nephrons increase filtration and eventually hypertrophy. Depending on the size of the remnant nephron pool, GFR will proportionately recover. If the number of remaining nephrons is below some critical number, continued hyperfiltration results in progressive glomerular sclerosis, eventually leading to nephron loss. A vicious cycle then ensues until complete renal failure occurs. This sequence explains the commonly observed scenario in which progressive renal failure occurs after initial recovery from acute renal failure.
CLINICAL FEATURES
Renal failure itself has few symptoms until severe uremia develops. Nausea, vomiting, drowsiness, fatigue, confusion, and coma are findings in uremia. Patients are more likely to present with symptoms related to the underlying cause of renal failure (Table 88-3), which should prompt assessment of renal function.7
| Risk Factor | Comments |
|---|---|
Medications
| Antibiotics, metformin, proton pump inhibitors Myopathy, rhabdomyolysis >90% renal excretion 66% renal excretion Low risk of renal tubular acidosis and nephrolithiasis Tacrolimus, cyclosporine |
Drugs of abuse
| |
Contrast agents
| Nephrogenic systemic fibrosis |
Systemic disease
| Drug effects; renal parenchymal invasion; bladder obstruction; light-chain proteins Rejection, infection |
Patients with prerenal acute renal failure commonly develop thirst, orthostatic light-headedness, and decreased urine output. Excessive vomiting, diarrhea, urination, hemorrhage, fever, or sweating can reduce circulating volume enough to precipitate acute renal failure. Causes of endothelial leak and third spacing, such as sepsis, pancreatitis, burns, and hepatic failure, can also result in prerenal failure, although these settings may also be associated with renal parenchymal injury. Progression of heart failure from any cause or overdiuresis of the patient with compensated congestive heart failure can result in renal failure. Decreased fluid intake from physical or cognitive disability can result in hypovolemia.
Intrinsic renal diseases can often be anticipated because of symptoms of the precipitating cause. Anticipate ischemic acute kidney injury after cardiac arrest, in severe sepsis, or with other causes of systemic hypotension. Renal failure from crystal-induced nephropathy, nephrolithiasis, and papillary necrosis can present as flank pain and hematuria. Suspect pigment-induced renal failure in rhabdomyolysis8 (see chapter 89, “Rhabdomyolysis”) or with hemolysis after recent blood transfusion. Darkening urine and edema with or without constitutional symptoms such as fever, malaise, and rash suggest acute glomerulonephritis, which may have been preceded by pharyngitis or cutaneous infection. Fever, arthralgia, and rash are common with acute interstitial nephritis. Acute renal arterial occlusion is usually marked by severe flank pain. Cough, dyspnea, and hemoptysis raise the possibility of Goodpasture’s syndrome or Wegener’s granulomatosis.
Suspect postrenal failure in men with prostatic disease or advanced age and patients with indwelling bladder catheters. Anuria strongly suggests obstruction, although vascular obstruction and fulminant renal disease are also possible. Alternating oliguria and polyuria is virtually pathognomonic of obstruction.
Assess and correct volume status. Evaluate mucous membranes, jugular vein distention, lung auscultation, peripheral edema, and tissue turgor to identify dehydration. Base deficit, lactate level, central venous pressure and oxygen saturation, and US (see Imaging) can be reliable indicators of hypovolemia. Carefully assess for rashes, evidence of vasculitis, jaundice, abdominal or pelvic masses, or a distended palpable urinary bladder. On cardiac exam, check for atrial fibrillation, abdominal aortic aneurysm, and signs of heart failure, and assess extremity pulses.
DIAGNOSIS
In the ED, the goals are to identify patients at risk for acute kidney injury who aren’t obviously ill and for those with diagnosed kidney injury to correct metabolic effects, decrease ongoing renal injury, and prevent iatrogenic injury. Determine if kidney injury is prerenal, postrenal, or intrinsic.
Obtain CBC, electrolyte levels including magnesium and phosphorus, and hepatic function tests and blood cultures if clinically appropriate. Obtain urinalysis, urine osmolality, and urine culture.
ECG is often the fastest screening test for hyperkalemia, but sensitivity for a level over 6.5 mmol/L ranges from 14% to 60%.9,10 Although ECGs were read as “abnormal” in 83% of patients in one study, peaked T waves were only seen in 34%, which led to delayed therapy for most patients for whom peaked T waves were not evident11 (see chapter 17, “Fluids and Electrolytes”). Chest radiography helps evaluate for increased volume, effusions, and pneumonia, all of which can result from or precipitate renal failure.
Obtain bedside US to assess urinary bladder volume. A large postvoid bladder residual volume (>125 mL) suggests bladder outlet obstruction for which you would place a urethral catheter. See chapter 92, “Acute Urinary Retention” for further discussion. Anuria is 100 mL urine per day and can be present with prerenal, postrenal, or intrinsic kidney disease. Alternating oliguria and polyuria is virtually pathognomonic of obstruction.
Creatinine (Cr) is the mainstay for measuring renal function; it is a breakdown product of the skeletal muscle protein creatine, and its level is thus linked to muscle mass. In patients with no renal function (GFR = 0), serum Cr level increases 1 to 3 milligrams/dL a day. Lesser increases in Cr indicate residual renal function, whereas faster increases suggest rhabdomyolysis. Elevation of serum Cr may take 48 hours to accumulate after onset of decreased function, and a patient with a very low baseline Cr level can lose more than half of the functioning nephrons before serum Cr elevates to an abnormal level.
Cr clearance is used to estimate GFR, and although it is not perfect, it is a useful measure in the ED. Patients with lower muscle mass (e.g., older patients and women) have lower actual GFRs for any given Cr level. Glomerulonephritis increases tubular secretion of Cr, but trimethoprim, cimetidine, and salicylates decrease tubular secretion of Cr, thus altering the Cr level independently of the GFR.
GFR calculations are provided online, for hand-held devices, and in electronic medical record systems, and there are several formulas for GFR calculation. Normal kidney function is a GFR >90 mL/min/1.73 m2, where 1.73 m2 is used as the average body surface area. Stages of kidney disease are characterized by GFR: stage 1, GFR 90 mL/min/1.73 m2; stage 2, GFR 60 to 89 mL/min/1.73 m2; stage 3, GFR 30 to 59 mL/min/1.73 m2; stage 4, GFR 15 to 29 mL/min/1.73 m2; and stage 5, GFR <15 mL/min/1.73 m2 (dialysis or transplant needed).