EPIDEMIOLOGY
Ischemic heart disease is the leading cause of death among adults in the United States, with more than 405,000 people dying annually. Atherosclerotic disease of the epicardial coronary arteries—termed coronary artery disease (CAD)—accounts for the vast majority of patients with ischemic heart disease. The predominant symptom of CAD is chest pain, and patient concern over potential acute heart disease contributes to the >8 million visits each year to U.S. EDs. In a typical adult ED population with acute chest pain, about 15% of patients will have an acute coronary syndrome (ACS). ACS encompasses unstable angina through acute myocardial infarction (AMI). Of patients with an ACS, approximately one third have an AMI, and the remainder have unstable angina.
The three principal presentations of unstable angina are listed in Table 49-1.1 These definitions assume that the anginal chest pain is due to ischemia, and this categorization does not apply to patients presenting to the ED with chest pain from other causes. During the initial ED assessment, it may not be possible to determine whether the patient has or will sustain permanent damage to the myocardium, has reversible ischemia (injury or unstable angina), or has a noncardiac cause of symptoms.
| Class | Presentation |
|---|---|
| Rest angina* | Angina occurring at rest and that is prolonged, usually >20 min |
| New-onset angina | New-onset angina that markedly limits ordinary physical activity, such as walking 1–2 blocks or climbing 1 flight of stairs or performing lighter activity |
| Increasing angina | Previously diagnosed angina that has become distinctly more frequent, has a longer duration, or is lower in threshold, limiting ability to walk 1–2 blocks or climb 1 flight of stairs or perform lighter activity |
The American College of Cardiology and American Heart Association have a tool for estimating the short-term risk for death or AMI in patients with unstable angina (Table 49-2).1
| Feature | High Likelihood (at least one of the following features must be present) | Intermediate Likelihood (no high-risk feature, but must have one of the following) | Low Likelihood (no high- or intermediate-risk feature, but may have any of the following) |
|---|---|---|---|
| History | Accelerating tempo of ischemic symptoms in preceding 48 h | Prior myocardial infarction, peripheral or cerebrovascular disease, or coronary artery bypass grafting; prior aspirin use | |
| Character of the pain | Prolonged, ongoing (>20 min) rest pain | Prolonged (>20 min) rest angina, now resolved, with moderate or high likelihood of CAD Rest angina (>20 min) or relieved with rest or sublingual nitroglycerin Nocturnal angina New-onset or progressive angina in the past 2 wk without prolonged (>20 min) rest pain but with intermediate or high likelihood of CAD (see Table 49-3) | Increased angina frequency, severity, or duration Angina provoked at a lower threshold New-onset angina with onset 2 wk to 2 mo before presentation |
| Clinical findings | Pulmonary edema, most likely due to ischemia New or worsening mitral regurgitation murmur S3 or new/worsening rales Hypotension, bradycardia, tachycardia Age >75 y old | Age >70 y old | Chest discomfort reproduced by palpation |
| ECG | Angina at rest with transient ST-segment changes >0.5 mm Bundle-branch block, new or presumed new Sustained ventricular tachycardia | T-wave changes, pathologic Q waves, or resting ST depression <1 mm in multiple lead groups (anterior, inferior, lateral) | Normal or unchanged ECG |
| Cardiac markers | Elevated cardiac TnT, TnI (e.g., TnT or TnI >0.1 nanogram/mL) | Slightly elevated cardiac TnT, TnI (e.g., TnT >0.01 but <0.1 nanogram/mL) | Normal |
ANATOMY
The left coronary artery divides into the left circumflex and the left anterior descending branches (Figure 49-1). The left anterior descending branch courses down the anterior aspect of the heart providing the main blood supply to the anterior and septal regions of the heart. The circumflex branch supplies blood to some of the anterior wall and a large portion of the lateral wall of the heart. The right coronary artery supplies the right side of the heart and provides some perfusion to the inferior aspect of the left ventricle through its continuation as the right posterior descending artery.
The atrioventricular conduction system receives blood supply from the atrioventricular branch of the right coronary artery and the septal perforating branch of the left anterior descending coronary artery. Similarly, the right bundle branch and the posterior division of the left bundle branch each obtain blood flow from both the left anterior descending and right coronary arteries. The posteromedial papillary muscle receives blood supply from one coronary artery, usually the right coronary artery.
PATHOPHYSIOLOGY
Ischemia occurs when there is an imbalance between oxygen (O2) demand and O2 supply. O2 supply is influenced by the O2-carrying capacity of the blood and the coronary artery blood flow. The O2-carrying capacity of the blood is determined by the amount of hemoglobin present and O2 saturation. Coronary artery blood flow is determined by the duration of diastolic relaxation of the heart and peripheral vascular resistance. Humoral, neural, metabolic, and extravascular compressive forces and local autoregulation mechanisms determine the coronary vascular resistance.
Exercise-induced myocardial ischemia and its sequelae usually occur as a result of fixed atherosclerotic lesions. ACS may be caused by secondary reduction in myocardial blood flow due to coronary arterial spasm, disruption or erosion of atherosclerotic plaques, and platelet aggregation or thrombus formation at the site of an atherosclerotic lesion. Secondary causes of myocardial ischemia are less common and prompted by factors extrinsic to the coronary arteries such as increased myocardial O2 demand (i.e., fever, tachycardia, thyrotoxicosis), reduced blood flow (i.e., hypotension), or reduced O2 delivery (i.e., anemia, hypoxemia). In the event of a secondary cause, global ischemia may occur widely or focally.
Atherosclerotic plaque forms through repetitive injury to the vessel wall. Macrophages and smooth muscle cells are the main cellular elements in plaque development, whereas lipids are predominant in the extracellular milieu. Plaque fissuring and rupture are affected by features inherent to the plaque, such as its composition and shape; local factors, such as shear forces, coronary arterial tone, and coronary arterial perfusion pressure; and movements of the artery in response to myocardial contractions. When plaque rupture occurs, potent thrombogenic substances activate circulating platelets.
The platelet response involves adhesion, activation, and aggregation. Platelet adhesion occurs through the weak platelet interactions with subendothelial adhesion molecules, such as collagen, fibronectin, and laminin, and the binding of the glycoprotein IIb receptor to the subendothelial form of von Willebrand factor. Adherent platelets are strongly thrombogenic. Lipid-laden macrophages in the plaque core and adventitia of the vessel wall release tissue factor, which stimulates the conversion of prothrombin to thrombin. Thrombin and the local shear forces are also potent platelet activators. Platelet secretion of adenosine diphosphate, thromboxane A2, and serotonin are autostimulatory agonists of platelet activation. Activated platelet glycoprotein IIb/IIIa receptors become cross-linked by fibrinogen or von Willebrand factor in the final common pathway of platelet aggregation.
The extent of O2 deprivation and the clinical presentation of ACS depend on the limitation of O2 delivery imposed by thrombus adhering to a plaque. In stable angina, ischemia occurs only when activity induces O2 demands beyond the supply restrictions imposed by a partially occluded coronary vessel. Ischemia occurs at a relatively fixed point and changes slowly over time. In this scenario, the atherosclerotic plaque has not ruptured, and there is little or no superimposed thrombus. In ACS, atherosclerotic plaque rupture and platelet-rich thrombus develop. Coronary blood flow is reduced suddenly, and myocardial ischemia occurs. The degree and duration of the O2 supply–demand mismatch determines whether the patient develops reversible myocardial ischemia without necrosis (unstable angina) or myocardial ischemia with necrosis (AMI). More severe and prolonged obstruction increases the likelihood of infarction.
AMI may inhibit myocardial contractility and impair both central and peripheral perfusion. When an area of the myocardium does not receive adequate O2, the functional deterioration progresses; as the size of the infarcted myocardium increases, left ventricular pump function decreases. This creates increased left ventricular end-diastolic pressure and end-systolic volume. Cardiac output, stroke volume, and blood pressure may decrease. When left atrial and pulmonary capillary pressures increase, heart failure or pulmonary edema may develop. Poor perfusion to the brain and kidneys can result in altered mental status and impaired renal function, respectively.
CLINICAL FEATURES
The main symptom of ischemic heart disease is chest discomfort or pain, and the history should characterize its severity, location, radiation, duration, and quality. In addition, the presence of associated symptoms such as nausea, vomiting, diaphoresis, dyspnea, light-headedness, syncope, and palpitations may help detect myocardial ischemia (see Tables 48-1 and 48-2 in chapter 48 “Chest Pain”). Obtain information regarding the onset and duration of symptoms, activities that precipitate symptoms, and prior evaluations for similar symptoms to assess the possibility of acute myocardial ischemia.
Symptoms of acute myocardial ischemia often will be described as discomfort rather than pain; look for descriptions of chest pressure, heaviness, tightness, fullness, or squeezing. Less commonly, patients will describe their symptoms as knife-like, sharp, or stabbing. The classic pain or discomfort location is substernal or in the left chest, with radiation to the arm (either), neck, or jaw. Reproducible chest wall tenderness is noted in some.
Exercise, stress, and a cold environment classically precipitate angina. Angina typically has a duration of symptoms of <10 minutes, occasionally lasting up to 10 to 20 minutes, and usually improves within 2 to 5 minutes after rest or nitroglycerin. In contrast, acute myocardial ischemia is usually accompanied by more prolonged and severe chest discomfort, more prominent associated symptoms (e.g., nausea, diaphoresis, shortness of breath), and little response to initial sublingual nitroglycerin. Easy fatigability may be a prominent symptom of ACS, especially in women.2
Ask about the frequency of anginal episodes and any change in frequency of episodes over the past months. Determine if there is any increase in severity or duration of symptoms, or whether less effort is required to precipitate symptoms.
Advanced age, female gender, and a history of diabetes mellitus are associated with more nonclassic ACS presentations. Presentations with nonclassic features or silent myocardial ischemia are common; for example, as many as 37.5% of women and 27.4% of men present without chest pain.3 Up to 30% of patients with acute myocardial ischemia identified in large longitudinal studies are clinically unrecognized, often not seeking medical care or not recalling any symptoms. The prognosis for patients with nonclassic symptoms (e.g., fatigue, weakness, not feeling well, vague discomfort) at the time of infarction is worse than that of patients with more classic symptoms. Women and the elderly are more likely to have presentations that differ from classic ones in the younger patients; across all age groups, those with unstable angina have nonclassic features nearly half the time. This is why the term atypical chest pain is misleading, because nonclassic presentations are common despite being often called atypical.
Cardiac risk factors are poor predictors of risk for AMI or other ACSs.4 Traditional cardiac risk factors for CAD, such as hypertension, diabetes mellitus, tobacco use, family history at an early age, and hypercholesterolemia, are not helpful to predict ACS in ED patients >40 years old.4 The cardiac risk factors predict risk of CAD over time, not likelihood of presence at one moment.
Patients with ACS may appear well, without any clinical signs of distress, or may be uncomfortable, pale, cyanotic, or in respiratory distress. The pulse rate may be normal or display bradycardia, tachycardia, or irregular pulses. Bradycardic rhythms are more common with inferior wall myocardial ischemia; in the setting of an acute anterior wall infarction, bradycardia or new heart block is a poor prognostic sign. Blood pressure can be normal, elevated (due to baseline hypertension, sympathetic stimulation, and anxiety), or decreased (due to pump failure or inadequate preload), although extremes of blood pressure are associated with a worse prognosis.
An S3 is present in 15% to 20% of patients with AMI; if detected, an S3 may indicate a failing myocardium. The presence of a new systolic murmur is an ominous sign, because it may signify papillary muscle dysfunction, a flail leaflet of the mitral valve with resultant mitral regurgitation, or a ventricular septal defect.
The presence of rales, with or without an S3 gallop, indicates left ventricular dysfunction and left-sided heart failure. Jugular venous distention, hepatojugular reflex, and peripheral edema suggest right-sided heart failure.
DIAGNOSIS
The diagnosis of ST-segment elevation myocardial infarction (STEMI) depends on the ECG in the setting of symptoms suggestive of myocardial infarction. The diagnosis of non-ST-segment elevation myocardial infarction (NSTEMI) depends on abnormal elevation of cardiac biomarkers but may include ECG changes not meeting criteria for STEMI. The diagnosis of unstable angina is based on history (Table 49-1) because the ECG and cardiac injury biomarkers are nondiagnostic. Early risk assessment for the likelihood of myocardial infarction uses all of these data to aid decision making (Tables 49-3 and 49-4).
| Feature | High Likelihood (any of the following) | Intermediate Likelihood (absence of high-likelihood features and presence of any of the following) | Low Likelihood (absence of high- or intermediate-likelihood features but may have the following) |
|---|---|---|---|
| History | Chest or left arm pain or discomfort as chief symptom reproducing prior documented angina Known history of coronary artery disease, including myocardial infarction | Chest or left arm pain or discomfort as chief symptom Age >70 y old Male sex Diabetes mellitus | Probable ischemic symptoms in absence of any of the intermediate-likelihood characteristics Recent cocaine use |
| Examination | Transient mitral regurgitation murmur, hypotension, diaphoresis, pulmonary edema, or rales | Extracardiac vascular disease | Chest discomfort reproduced by palpation |
| ECG | New, or presumably new, transient ST-segment deviation (≥1 mm) or T-wave inversion in multiple precordial leads | Fixed Q waves ST depression 0.5–1.0 mm or T-wave inversion >1 mm | T-wave flattening or inversion <1 mm in leads with dominant R waves Normal ECG |
| Cardiac markers | Elevated cardiac troponin I, troponin T, or MB fraction of creatine kinase | Normal | Normal |
Age 65 y or older 3 or more traditional risk factors for coronary artery disease Prior coronary stenosis of 50% or more ST-segment deviation on presenting electrocardiogram 2 or more anginal events in prior 24 h Aspirin use within the 7 d prior to presentation Elevated cardiac markers The presence of each of the above is assigned 1 point. The maximum possible score is 7. |
The Thrombosis in Myocardial Infarction (TIMI) score is a seven-item tool that helps stratify patients with potential ACSs in the ED. Patients with a score of 0 to 2 have a 2% to 9% 30-day risk of death, myocardial infarction, or revascularization. Patients with higher scores have higher risks.
The standard 12-lead ECG is the single best test—although it can be fallible—to identify patients with AMI upon ED presentation.1 Obtain the initial 12-lead ECG and interpret the tracing quickly, ideally within 10 minutes of presentation in those patients with symptoms suggestive of myocardial ischemia. Prehospital ECGs reduce the time from symptom onset to reperfusion therapy in STEMI patients and are an optimal tool when possible.5,6
ST-segment–based diagnostic ECG criteria for AMI are shown in Table 49-5. STEMI in the listed distributions suggests acute transmural injury. ST-segment depressions in these distributions suggest ischemia. Inferior wall AMIs should have a right-sided lead V4 (V4R) obtained, because ST-segment elevation in V4R is highly suggestive of right ventricular infarction. For patients with a nondiagnostic tracing and persistent symptoms who have a high risk of ACS, repeat the ECG to detect developing changes.1,5,7 Early studies of fibrinolytic therapy identified an increased risk of mortality in patients with new bundle-branch block; this led to interpreting a new left bundle-branch block as being a “STEMI equivalent.” However, <10% of patients with new or possibly new left bundle-branch block have AMI.8
| Location | Electrocardiographic Findings |
|---|---|
| Anteroseptal | ST-segment elevations in V1, V2, and possibly, V3 |
| Anterior | ST-segment elevations in V1, V2, V3, and V4 |
| Anterolateral | ST-segment elevations in V1–V6, I, and aVL |
| Lateral | ST-segment elevations in I and aVL |
| Inferior | ST-segment elevations in II, III, and aVF |
| Inferolateral | ST-segment elevations in II, III, aVF, and V5 and V6 |
| True posterior* | Initial R waves in V1 and V2>0.04 s and R/S ratio ≥1 |
| Right ventricular | ST-segment elevations in II, III, and aVF and ST elevation in right-side V4 |
Reciprocal ST-segment changes—those in leads away from or opposite the elevation area—are from subendocardial ischemia and denote a larger area of injury risk, an increased severity of underlying CAD, more severe pump failure, a higher likelihood of cardiovascular complications, and increased mortality. In general, the more elevated the ST segments and the more ST segments that are elevated, the more extensive is the injury.
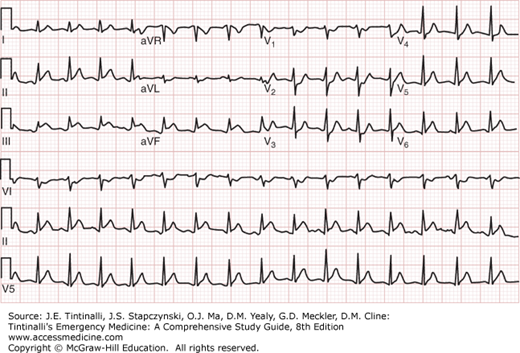
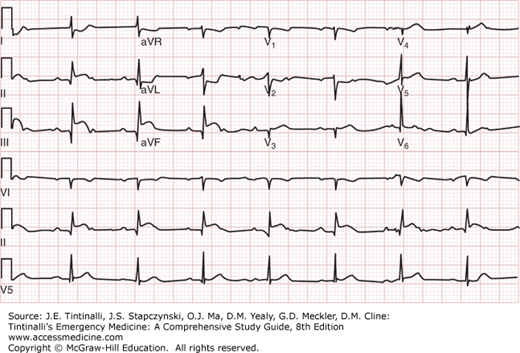
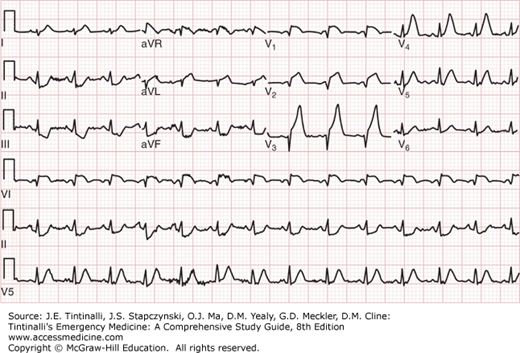
The ECG changes correlate often with the infarct-related vessel (Table 49-6). Inferior wall AMIs can result from occlusion of the left circumflex artery or the right coronary artery. In the setting of an inferior wall AMI, ST-segment elevation in at least one lateral lead (V5, V6, or aVL) with an isoelectric or elevated ST segment in lead I is strongly suggestive of a left circumflex lesion (Figure 49-2). The presence of ST-segment elevation in lead III greater than that in lead II predicts a right coronary artery occlusion (Figure 49-3). When accompanied by ST-segment elevation in V1 or a V4R, it predicts a proximal right coronary artery lesion with accompanying right ventricular infarction (Figure 49-4). Reciprocal anterior ST-segment depressions in V1 through V4 are equally prevalent in right coronary and left circumflex inferior wall AMIs. Figure 49-5 shows anterior myocardial infarction from distal left anterior descending artery occlusion, whereas Figure 49-6 shows anterior myocardial infarction from proximal left anterior descending artery occlusion.
| ECG Findings | Culprit Artery | Sensitivity (%) | Specificity (%) | Positive Predictive Value (%) | Negative Predictive Value (%) |
|---|---|---|---|---|---|
| ECG findings for inferior ST-segment elevation myocardial infarction | |||||
| ST-segment elevation in lead III greater than in lead II plus ST-segment depression of >1 mm in lead I, lead aVL, or both | Right coronary artery | 90 | 71 | 94 | 70 |
| In addition to the findings immediately above, ST-segment elevation on V1, V4R, or both | Proximal right coronary artery | 79 | 100 | 100 | 88 |
| Absence of the above findings plus ST-segment elevation in leads I, aVL, V5, and V6 and ST-segment depression in leads V1, V2, and V3 | Left circumflex coronary artery | 83 | 96 | 91 | 93 |
| ECG findings for anterior ST-segment elevation myocardial infarction | |||||
| ST-segment elevation in leads V1, V2, and V3 plus any of the features below: | |||||
| ST-segment elevation of >2.5 mm in lead V1, or right bundle-branch block with Q wave, or both | Proximal left anterior descending coronary artery | 12 | 100 | 100 | 61 |
| ST-segment depression of >1 mm in leads II, III, and aVF | Proximal left anterior descending coronary artery | 34 | 98 | 93 | 68 |
| ST-segment depression of ≤1 mm, or ST-segment elevation in leads II, III, and aVF | Distal left anterior descending coronary artery | 66 | 73 | 78 | 62 |
FIGURE 49-2.
ECG showing inferior-lateral myocardial infarction from left circumflex coronary artery occlusion. ECG from a 42-year-old man presenting with chest pain. ECG shows ST-segment elevation in limb leads II, III (inferior), and aVF, as well as lead V6 (lateral). ST-segment depression is evident in leads V1, V2, and V3, reflecting reciprocal changes in the anterior leads. The patient was found to have 100% occlusion of the left circumflex coronary artery at cardiac catheterization. [Used with permission of David M. Cline, MD, Wake Forest University.]
FIGURE 49-3.
ECG showing inferior myocardial infarction from right coronary artery occlusion. ECG from an 80-year-old man presenting with acute chest pain. The ECG shows ST-segment elevation in lead III greater than in lead II plus ST-segment depression of >1 mm in lead I and lead aVL. The patient was found to have 100% occlusion of the right coronary artery at cardiac catheterization. [Used with permission of David M. Cline, MD, Wake Forest University.]
FIGURE 49-4.
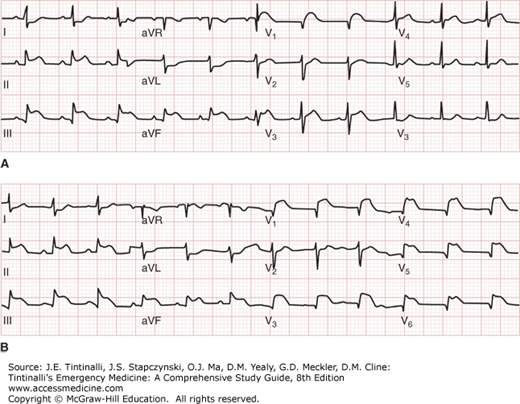
A. Inferior wall myocardial infarction with ST elevation in lead V1. ECG showing inferior ST-segment elevation myocardial infarction and ST-segment elevation in lead V1 suggestive of right ventricular infarction. B. Inferior wall myocardial infarction with right ventricular leads. Same patient with placement of right ventricular leads, showing ST-segment elevation in V3R, V4R, V5R, and V6R compatible with right ventricular infarction. [Used with permission of J. Stephan Stapczynski, Maricopa Medical Center.]
FIGURE 49-5.
ECG showing anterior myocardial infarction from distal left anterior descending coronary artery occlusion. ECG from a 52-year-old man presenting with chest pain. ECG shows ST-segment elevation in V1, V2, and V3, with the absence of ST-segment depression in leads II, III, and aVF. The patient was found to have 100% occlusion of the distal left anterior descending coronary artery at cardiac catheterization. [Used with permission of David M. Cline, MD, Wake Forest University.]
FIGURE 49-6.
ECG showing anterior myocardial infarction from proximal left anterior descending coronary artery occlusion. ECG from a 65-year-old man presenting with chest pain. ECG shows ST-segment elevation in V1, V2, and V3, and >1 mm of ST-segment depression in leads II, III, and aVF. The patient was found to have 100% occlusion of the proximal left anterior descending coronary artery at cardiac catheterization. [Used with permission of David M. Cline, MD, Wake Forest University.]
ECGs are frequently misinterpreted, with a low of 5.9% to as many as 29% being misinterpreted.14 False-positive interpretations of the ECG, indicating STEMI when no injury exists, occur between 11% and 14% of the time.13 Balancing this is the observation that even patients with normal or nonspecific ECGs have a 1% to 5% incidence of AMI and a 4% to 23% incidence of unstable angina. Patients with nondiagnostic ECGs or evidence of ischemia that is age-indeterminate have a 4% to 7% incidence of AMI and a 21% to 48% incidence of unstable angina. Demonstration of new ischemia in ECG increases the risk of AMI from 25% to 73% and the unstable angina risk from 14% to 43%. Thus, the standard 12-lead ECG is useful for cardiovascular risk stratification of patients with ACSs. The only guideline-recommended addition to the standard 12-lead ECG is the use of right-sided precordial lead, V4R, in the setting of acute inferior myocardial infarction to detect right ventricular involvement.1,5,6
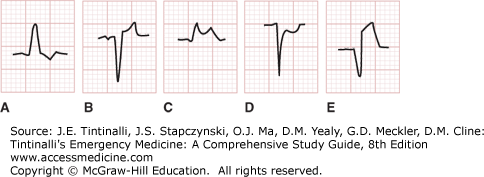
There are several clinical conditions in which ECG interpretation is difficult (Table 49-7). In the setting of paced rhythms or left bundle-branch block, acute myocardial ischemia can be identified (Figure 49-7) with select findings. In those with a preexisting left bundle-branch block, the following patterns are indicative of AMI: (1) ST-segment elevation of 1 mm or greater and concordant (in the same direction as the main deflection) with the QRS complex (odds ratio, 25.2; 95% confidence interval [CI], 11.6% to 54.7%) seen in Figure 49-7C; (2) ST-segment depression of 1 mm or more in leads V1, V2, or V3 (odds ratio, 6.0; 95% CI, 1.9% to 19.3%) seen in Figure 49-7D; and (3) ST-segment elevation of 5 mm or greater and discordant (in the opposite direction) with the QRS complex (odds ratio, 4.3; 95% CI, 1.8% to 10.6%) seen in Figure 49-7E.14
May have ST-segment elevation in the absence of acute myocardial infarction Early repolarization Left ventricular hypertrophy Pericarditis Myocarditis Left ventricular aneurysm Hypertrophic cardiomyopathy Hypothermia Ventricular paced rhythms Left bundle-branch block May have ST-segment depressions in the absence of ischemia Hypokalemia Digoxin effect Cor pulmonale and right heart strain Early repolarization Left ventricular hypertrophy Ventricular-paced rhythms Left bundle-branch block May have T-wave inversions in the absence of ischemia Persistent juvenile pattern Stokes-Adams syncope or seizures Posttachycardia T-wave inversion Postpacemaker T-wave inversion Intracranial pathology (CNS hemorrhage) Mitral valve prolapse Pericarditis Primary or secondary myocardial diseases Pulmonary embolism or cor pulmonale from other causes Spontaneous pneumothorax Myocardial contusion Left ventricular hypertrophy Ventricular-paced rhythms Left bundle-branch block Right bundle-branch block |
FIGURE 49-7.
Discordant and concordant ST elevation and depression in the setting of left bundle-branch block. ST-segment abnormalities in left bundle-branch block. A. Discordant ST-segment depression (“normal”). B. Discordant ST-segment elevation (“normal”). C. Concordant ST-segment elevation (strongly suggestive of acute myocardial infarction [AMI]). D. Concordant ST-segment depression (suggestive of AMI). E. Excessive (>5 mm) discordant ST-segment elevation (weakly suggestive of AMI). [Used with permission of William Brady, MD, University of Virginia.]
Right ventricular pacing, the common pacemaker lead location, causes secondary repolarization changes of opposing polarity to that of the predominant QRS complex. Most leads have predominant negative QRS complexes followed by ST-segment elevation and positive T waves. ST-segment elevation of at least 5 mm is most indicative of AMI in leads with predominantly negative QRS complexes.15 Any ST-segment elevation concordant to the QRS complex in a predominantly positive QRS complex is highly specific for AMI. The QRS complex is predominantly negative in leads V1 to V3 with right ventricular pacing. ST-segment depression in these leads has 80% specificity for AMI.15
Patients with diagnostic ST-segment elevation on their initial ECG do not require serum marker measurement to make treatment and disposition decisions. Conversely, serum markers are useful in patients with nondiagnostic ECGs for diagnosis of NSTEMI and risk stratification of patients with STEMI, NSTEMI, and unstable angina. Even low-level cardiac marker elevations are independent risk factors for acute (<30 days) cardiac complications and short-term (<1 year) prognosis in unstable angina.16 A rise in serum troponin I or T, with at least one value above the 99th percentile, is diagnostic for AMI in patients with symptoms consistent with ACS.17 Low-level elevations in either troponin correlate with risk for cardiovascular complications in unstable angina, CAD, and renal failure.16 Troponin is sensitive and specific for cardiac myocardial necrosis, but there are many causes of myocardial necrosis unrelated to ACS (see Table 48-5). Minor elevations in cardiac troponin I and cardiac troponin T identify patients more likely to benefit from treatment with glycoprotein IIb/IIIa inhibitors, enoxaparin, and early invasive treatment strategy (catheterization within 24 to 48 hours).18
New high-sensitivity cardiac troponins have improved ability to detect ischemia. First-generation single assays of cardiac troponin I at the time of presentation had an AMI sensitivity of 39%. Serial sampling increased sensitivity to 90% to 100%, with specificity of 83% to 96% for cardiac troponin I and 76% to 91% for cardiac troponin T. The high-sensitivity troponins, initially used in Europe, identify 90% to 100% of patients with AMI at the time of arrival using the lowest cut point, albeit with limited specificity (between 34% and 80% depending on the cut-off used).19,20,21 The concern regarding use of high-sensitivity assays focuses on the frequency of false-positive results that lead to unnecessary procedures and hospital admissions or no benefit. Authors of a large observational study advocated using a single undetectable high-sensitivity troponin plus no ECG evidence of ischemia as a decision point to discharge chest pain patients from the ED.
Despite the new sensitive assays, guidelines1,5,6 and experts22 recommend serial troponin testing to identify acute disease. A serial high-sensitivity troponin interval as short as 2 hours coupled with a low TIMI risk score (<2) virtually excludes AMI.23 The European Society of Cardiology recommends a 3-hour serial interval when high-sensitivity troponins are used.24
Elevated levels of the cardiac troponins in patients with NSTEMI increase the short-term risk of death 3.1-fold (1.6% vs 5.2%).19 Although patients with elevated troponins in the absence of ACS may be “false positive” for AMI, elevated troponin of any amount is associated with a high frequency of worse outcomes. Taken together, the data strongly support the claim that any measurable elevated troponin is always worse than no elevated troponin and that more troponin elevation is always worse than less troponin elevation with respect to prognosis.
B-type natriuretic peptide, an established marker for patients with heart failure, is also elevated in patients with ACS and can identify ACS patients who are at higher risk for adverse cardiovascular events, heart failure, or death.5 When used in conjunction with other markers, the addition of B-type natriuretic peptide increases sensitivity at the cost of decreased specificity, with only a slightly increase in overall diagnostic accuracy. For this reason, B-type natriuretic peptide is not routinely needed in those with suspected ACS.
GENERAL TREATMENT
The treatment of ACSs is based on duration and persistence of symptoms, cardiac history, and findings on physical examination and the initial ECG (see Figure 49-8). Establish IV access, give aspirin if not already given by EMS, and, as long as there are no contraindications, provide ECG monitoring. Many recommend supplemental O26; however, there is little evidence for benefit in patients without hypoxemia, and small studies have shown a negative effect with high-flow O2.25 The key treatment strategies aim to achieve immediate reperfusion and limit infarct size (Tables 49-8 and 49-9).
| Antiplatelet Agents | ||
| Aspirin | 162–325 milligrams. | |
| Clopidogrel | Loading dose of 600 milligrams PO followed by 75 milligrams/d. No loading dose is administered in patients >75 y old receiving fibrinolytics. | |
| Prasugrel | Loading dose of 60 milligrams promptly and no more than 1 h after PCI once coronary anatomy is defined and a decision is made to proceed with PCI. | |
| Ticagrelor | Loading dose is 180 milligrams PO followed by 90 milligrams twice a day. | |
| Antithrombins | ||
| Unfractionated heparin | Bolus of 60 units/kg (maximum, 4000 units) followed by infusion of 12 units/kg/h (maximum, 1000 units/h) titrated to a partial thromboplastin time 1.5–2.5 × control. | |
| Enoxaparin | 30 milligrams IV bolus followed by 1 milligram/kg SC every 12 h. | |
| Fondaparinux | 2.5 milligrams SC.* | |
| Fibrinolytic Agents | ||
| Streptokinase | 1.5 million units over 60 min. | |
| Anistreplase | 30 units IV over 2–5 min. | |
| Alteplase | Body weight >67 kg: 15 milligrams initial IV bolus; 50 milligrams infused over next 30 min; 35 milligrams infused over next 60 min. Body weight <67 kg: 15 milligrams initial IV bolus; 0.75 milligrams/kg infused over next 30 min; 0.5 milligram/kg infused over next 60 min. | |
| Reteplase | 10 units IV over 2 min followed by 10 units IV bolus 30 min later. | |
| Tenecteplase | Weight | Dose (total dose not to exceed 50 milligrams) |
| <60 kg | 30 milligrams | |
| ≥60 but <70 kg | 35 milligrams | |
| ≥70 but <80 kg | 40 milligrams | |
| ≥80 but <90 | 45 milligrams | |
| ≥90 | 50 milligrams | |
| Glycoprotein IIb/IIIa Inhibitors† | ||
| Abciximab | 0.25 milligram/kg bolus followed by infusion of 0.125 microgram/kg/min (maximum, 10 micrograms/min) for 12–24 h. | |
| Eptifibatide | 180 micrograms/kg bolus followed by infusion of 2.0 micrograms/kg/min for 72–96 h. | |
| Tirofiban | 0.4 micrograms/kg/min for 30 min followed by infusion of 0.1 microgram/kg/min for 48–96 h. | |
| Other Anti-Ischemic Therapies | ||
| Nitroglycerin | Sublingual: 0.4 milligram every 5 min × 3 PRN pain. Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

| |