INTRODUCTION AND EPIDEMIOLOGY
Asthma is a chronic inflammatory disorder characterized by increased responsiveness of the airways to multiple stimuli. In susceptible individuals, the inflammation causes recurrent episodes of wheezing, breathlessness, chest tightness, and coughing, particularly at night or in the early morning. These episodes usually are associated with widespread and varying airflow obstruction.
Although most acute attacks are reversible and improve spontaneously or within minutes to hours with treatment with symptom-free intervals in between, many asthmatic patients develop chronic airflow limitation. This impacts the diagnosis of, management of, and attempts to prevent acute exacerbations.
Asthma affects approximately 8% of the U.S. population, is the most common chronic disease of childhood (9% prevalence), affects 7% of the elderly, and has a similar prevalence in developed nations around the world.1,2,3 Approximately one half of cases of asthma develop before the age of 10 years old, and another one third develop before the age of 40 years old.
PATHOPHYSIOLOGY
Asthma is characterized by an abnormal accumulation of eosinophils, lymphocytes, mast cells, macrophages, dendritic cells, and myofibroblasts in airways. The pathophysiologic hallmark of asthma is a reduction in airway diameter caused by smooth muscle contraction, vascular congestion, bronchial wall edema, and thick secretions. These changes are reflected in pulmonary function changes, increased work of breathing, and abnormal distribution of pulmonary blood flow (Table 69-1). Large and small airways contain plugs composed of mucus, serum proteins, inflammatory cells, and cellular debris. On a microscopic level, airways are infiltrated with eosinophils and mononuclear cells. Evidence of microvascular leakage, epithelial disruption, and vasodilation is frequently noted. The airway smooth muscle is hypertrophied and characterized by new vessel formation, an increased number of epithelial goblet cells, and deposition of interstitial collagen beneath the epithelium. Inflammation affects all bronchial pulmonary structures.
Increased airway resistance Decreased maximum expiratory flow rates Air trapping Increased airway pressure Barotrauma Adverse hemodynamic effects Ventilation–perfusion imbalance Hypoxemia Hypercarbia Increased work of breathing Pulsus paradoxus Respiratory muscle fatigue with ventilatory failure |
Asthma is a continuum from acute bronchospasm to airway inflammation to permanent airway remodeling. The structural changes associated with airway remodeling, such as sub–basement membrane thickening, subepithelial fibrosis, airway smooth muscle hypertrophy and hyperplasia, angiogenesis, and mucous gland hyperplasia and hypersecretion are associated with nonreversible loss of lung function.4 Acute allergic bronchoconstriction results from immunoglobulin E–dependent release of mediators from mast cells. These mediators include histamine, leukotrienes, tryptase, and prostaglandins that directly contract airway smooth muscle.4 Bronchospasm induced by aspirin and other nonsteroidal anti-inflammatory drugs also involves mediator release from airway cells.4
Inflammation plays a key role in the pathophysiology of asthma regardless of disease severity. Inhaled antigens activate immunoglobulin E, mast cells, and T helper cells in the airway and induce the production of inflammatory mediators and cytokines. In turn, this initiates a cascade of reactions involving lymphocytes, mast cells, eosinophils, dendritic cells, macrophages, resident airway cells, and epithelial cells that perpetuate the inflammatory response, with further release of chemokines, cytokines, cysteinyl leukotrienes, and nitric oxide. The inflammatory process is multicellular, redundant, and self-amplifying.
Numerous host and environmental factors, such as number and type of infections in childhood, frequent antibiotic use, Western lifestyle, and repeated exposures to allergens, may contribute to the development of allergic asthma. Viral respiratory infections are among the most common of the stimuli that invoke acute asthma exacerbation.5 Increased airway responsiveness secondary to infection may last anywhere from 2 to 8 weeks.5 Exercise is another common precipitant of acute asthma. Environmental conditions, such as atmospheric pollutants and antigens noted in heavy industrial or densely populated urban areas, or increased indoor antigens, such as mold, house dust mites, cockroaches, and animal dander, are associated with higher incidence and severity of asthma. Occupational exposures, such as metal salt, wood and vegetable dust, pharmaceuticals, industrial chemicals, plastics, biological enzymes, vapors, gases, and aerosols, also may stimulate an asthma attack. Agents such as aspirin, β-blockers (including topical β-blockers), nonsteroidal anti-inflammatory drugs, sulfating agents, tartrazine dyes, and food additives and preservatives may trigger acute asthma. Exposure to cold air alone can induce acute bronchospasm. Endocrine factors, such as changing levels of estradiol and progesterone during the normal menstrual cycle and pregnancy, contribute to the level of airway reactivity.6 Emotional stress also can produce an asthma attack.
CLINICAL FEATURES
The symptoms of asthma include dyspnea, wheezing, and cough. Many, but not all, patients will relay the history of asthma upon presentation. Early in the attack, patients will complain of a sensation of chest constriction and cough. As the exacerbation progresses, wheezing becomes apparent, expiration becomes prolonged, and accessory muscle use may ensue. A thorough history can be helpful in guiding care for asthma exacerbations7,8,9,10 (Table 69-2). Acute asthma exacerbations are categorized based on clinical features7,8,9,10 (Table 69-3).
| Symptoms | Pattern | Disease History | Risk Factors for Death from Asthma |
|---|---|---|---|
| Cough | Perennial and/or seasonal | Age at onset | Past history of severe exacerbation |
| Wheezing | Continual or episodic | Present management and medications | ≥2 hospitalizations for asthma in the past year |
| Shortness of breath | Onset | Medication regimen adherence | >3 ED visits for asthma in the past year |
| Chest tightness | Duration | History of corticosteroid use (chronic and/or intermittent) | >2 canisters per month of inhaled short-acting β2-agonist |
| Sputum production | Frequency | Intensive care admissions | Difficulty perceiving airflow obstruction or its severity |
| Fever | Aggravating factors | History of intubation | Low socioeconomic status or inner-city resident |
| Usual pattern of exacerbation and outcome | Best spirometry measures | Illicit drug use | |
| Psychiatric disease or medical comorbidities |
| Symptoms and Signs | Initial PEF (or FEV1) | Clinical Course | |
|---|---|---|---|
| Mild | Dyspnea only with activity | PEF ≥70% predicted or personal best | Prompt relief with inhaled SABA. |
| Moderate | Dyspnea interferes with or limits usual activity | PEF 40%–69% predicted or personal best | Relief from frequent inhaled SABA. Symptoms for 1–2 d after oral corticosteroids begun. |
| Severe | Dyspnea at rest; interferes with conversation | PEF <40% predicted or personal best | Partial relief from frequent inhaled SABA. Symptoms for ≥3 d after oral corticosteroids begun. |
| Subset: life-threatening | Too dyspneic to speak; perspiring | PEF <25% predicted or personal best | Minimal or no relief from frequent inhaled SABA; IV steroids; adjunctive therapy; needs ED or intensive care unit. |
Physical examination findings are variable. Patients presenting with a severe asthma attack may be in respiratory distress, with rapid breathing and loud wheezing, whereas patients with mild exacerbation may present with only cough and end-expiratory wheezing. At times, wheezing may be audible without a stethoscope. Other conditions may present with wheezing and mimic asthma (Table 69-4). The use of accessory muscles of inspiration indicates diaphragmatic fatigue. The appearance of paradoxical respiration, which is chest deflation and abdominal protrusion during inspiration followed by chest expansion and abdominal deflation during expiration, is a sign of impending ventilatory failure. Alteration in the mental status (e.g., lethargy, exhaustion, agitation, or confusion) also heralds respiratory arrest.
Directed physical examination reveals hyperresonance to percussion, decreased intensity of breath sounds, and prolongation of the expiratory phase, usually with wheezing. Although wheezing results from the movement of air through narrowed airways, the intensity of the wheeze may not correlate with the severity of airflow obstruction. The “silent chest” reflects very severe airflow obstruction, with air movement insufficient to promote an audible wheeze. A pulsus paradoxus (change in blood pressure during inspiration) >20 mm Hg is also indicative of severe asthma, although it is not specific for asthma. Although tachycardia and tachypnea are usually seen with acute asthma, a normal heart rate, a normal respiratory rate, and the absence of a pulsus paradoxus do not indicate complete relief of airway obstruction.
Bedside spirometry provides a rapid, objective assessment of patients and guides therapy. The forced expiratory volume in 1 second (FEV1) and the peak expiratory flow rate (PEFR) rate directly measure the degree of large airway obstruction. Sequential measurements help assess severity and determine response to therapy. A flow-volume loop can help distinguish asthma from vocal cord dysfunction; the latter is often treated as asthma, sometimes with repeated visits. Vocal cord dysfunction responds to the moisture, not the drug, in a nebulized treatment, and “fails” metered-dose inhaler outpatient therapy because moisture is not a part of that delivery system. If not documented, obtain a full spirometry assessment only in patients with frequent outpatient failures.
Signs on physical examination and the subjective symptoms do not necessarily correlate well with the severity of airflow obstruction, making objective measures valuable. Patient cooperation is essential for these tests to be reliable, limiting the value of spirometry in severe exacerbations or in noncooperative patients.7,8,9,10
Pulse oximetry assesses oxygen saturation during treatment. Arterial blood gas measurement is not needed in most patients with mild to moderate asthma exacerbation, and it should be reserved for suspected hypoventilation with carbon dioxide retention and respiratory acidosis. With acute attacks, ventilation is stimulated, resulting in a decrease in partial pressure of arterial carbon dioxide (Paco2). Therefore, a normal or slightly elevated Paco2 (e.g., >42 mm Hg) indicates extreme airway obstruction and fatigue and may herald the onset of acute ventilatory failure. Patients with impending respiratory failure almost always have clinical evidence of severe attacks or spirometry demonstrating a PEFR or FEV1 <25% predicted.11 The use of capnography in acute asthma management is unclear. One small study reported good concordance between expired carbon dioxide levels measured by capnography and arterial carbon dioxide concentration,12 but another reported differences of up to 10 mm Hg or more between the two measurements.13
Radiography is indicated only if there is clinical suspicion of pneumothorax, pneumomediastinum, pneumonia, or other cause for symptoms (e.g., acute heart failure) or complication of asthma. For admitted asthma patients, less than one third of patients have an abnormal chest radiograph.14
A CBC is not routinely needed and likely will show modest leukocytosis secondary to administration of β-agonist therapy or corticosteroids. For those few patients taking theophylline, measure the serum level. Routine ECG is also unnecessary but may reveal right ventricular strain, abnormal P waves, or nonspecific ST- and T-wave abnormalities, which resolve with treatment. Older patients, especially those with coexisting heart disease, should have cardiac monitoring during treatment.
STANDARD TREATMENT
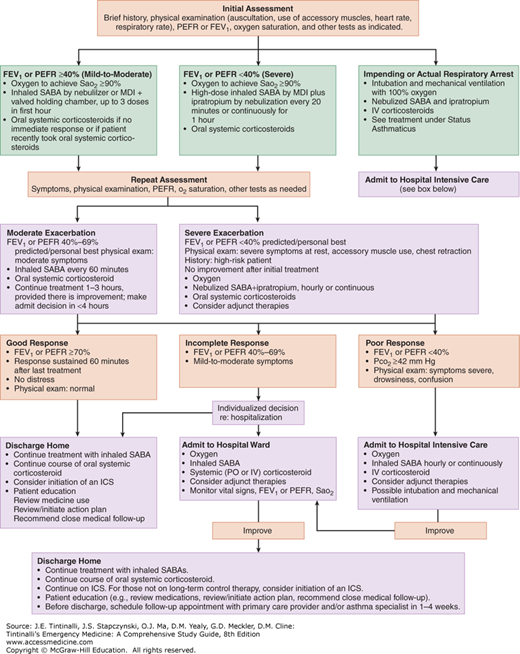
The goal is rapid reversal of airflow obstruction by repetitive or continuous administration of inhaled β2-agonists, ensuring adequate oxygenation, and relieving inflammation.7,8,9,10 Figure 69-1 shows the National Asthma Education and Prevention Program Expert Panel ED treatment algorithm.10 The following categories of medications are used in the treatment of acute asthma: β-adrenergic agonists, anticholinergics, and glucocorticoids. Treatments for impending or actual respiratory arrest are discussed below in “Status Asthmaticus.”
FIGURE 69-1.
Management of asthma exacerbations: ED and hospital-based care. FEV1 = forced expiratory volume in 1 second; ICS = inhaled corticosteroid; MDI = metered-dose inhaler; PEFR = peak expiratory flow rate; SABA = short-acting β2-agonist; Sao2 = oxygen saturation by pulse oximetry. [Source: (National Heart, Lung, and Blood Institute; National Institutes of Health; U.S. Department of Health and Human Services: National Asthma Education and Prevention Program, Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Publication No. 08-4051. Bethesda, MD, National Institutes of Health, 2007.) Accessed November 18, 2014.]
β-Adrenergic agonists with rapid onset are the preferred initial rescue medication for acute bronchospasm (Table 69-5). Stimulation of β1-receptors increases rate and force of cardiac contraction and decreases small intestine motility and tone, whereas β2-adrenergic stimulation promotes bronchodilation, vasodilation, uterine relaxation, and skeletal muscle tremor.