7.35, alkalemia refers to a pH ![]() 7.45. Acidosis refers to a process that increases hydrogen ion concentration, while alkalosis refers to a process that decreases hydrogen ion concentration. Patients are either acidemic or alkalemic, but can have multiple simultaneous acid–base processes.
7.45. Acidosis refers to a process that increases hydrogen ion concentration, while alkalosis refers to a process that decreases hydrogen ion concentration. Patients are either acidemic or alkalemic, but can have multiple simultaneous acid–base processes.
The following is a basic approach for ABG interpretation.
Step 1: Look at pH and PaCO2 to determine the primary disorder. The calculated HCO3− should be examined to ensure proper interpretation:
Step 2: Determine if appropriate compensation is present (based on formulas specific to each disorder). Remember that compensation never fully corrects the pH (see Table 44.1).
Step 3: If degree of compensation is not appropriate, determine whether multiple disorders are present.
- Metabolic acidosis or alkalosis:
- PaCO2 lower than expected = concomitant respiratory alkalosis.
- PaCO2 higher than expected = concomitant respiratory acidosis.
- PaCO2 lower than expected = concomitant respiratory alkalosis.
- Respiratory acidosis or alkalosis:
- pH lower than expected = concomitant metabolic acidosis.
- pH higher than expected = concomitant metabolic alkalosis.
- pH lower than expected = concomitant metabolic acidosis.
Step 4: If a metabolic acidosis is present, determine whether it is an anion-gap metabolic acidosis. If an anion-gap metabolic acidosis is present, calculate the delta-delta gap (see below).
Step 5: If normal pH, consider other possibilities:
- High PaCO2 and high HCO3− : respiratory acidosis + metabolic alkalosis.
- Low PaCO2 and low HCO3−: respiratory alkalosis + metabolic acidosis.
- Normal PaCO2 and HCO3−, but elevated anion gap: anion gap metabolic acidosis + metabolic alkalosis.
- Normal PaCO2, HCO3−, and anion gap: no acid–base disturbance, or non–anion-gap acidosis + metabolic alkalosis.
Table 44.1. Compensation formulas for primary acid–base disorders
| Primary disorder | Expected compensation |
| Metabolic acidosis | 1. Expected PaCO2 = 1.5 × [HCO3−] + 8 ±2 (Winter’s formula) 2. Alternatively, the last two digits of pH should approximate PaCO2 |
| Metabolic alkalosis | PaCO2 = 0.7 × [HCO3−] + 20 ±5 |
| Respiratory acidosis | 1. Acute: 2. Chronic: |
| Respiratory alkalosis | 1. Acute: 2. Chronic: |
Metabolic acidosis
Presentation
Classic presentation
- Symptoms depend on the severity and etiology of the underlying acidosis, and are often nonspecific. Altered mental status, weakness, nausea, and abdominal pain are common.
- Hyperkalemia is often present due to transcellular shift of K+ out of cells and H+ into cells.
- Kussmaul respirations are classically associated with diabetic ketoacidosis (DKA), and refer to rapid, deep breathing.
Critical presentation
- Extreme acidemia leads to neurological dysfunction (severe obtundation, coma, and seizures) as well as cardiovascular complications (arrhythmias, decreased cardiac contractility, arteriolar vasodilation, and decreased responsiveness to catecholamines). Profound hypotension and shock can result, which can complicate management since hypotension and shock are often the cause of the acidosis.
Diagnosis and evaluation
History and physical examination
- History and physical are generally revealing (e.g., a patient with a history of DKA who presents with nausea, weakness, and signs of dehydration).
Diagnostic tests
- Calculate the anion gap (Na+ − [Cl− + HCO3−]). The expected anion gap is 2.5 × [Albumin].
- If an elevated anion gap (AG) is present, check delta-delta gap (
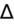
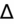 ) to evaluate for secondary metabolic derangement. The basic concept behind the
) to evaluate for secondary metabolic derangement. The basic concept behind the 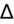
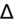 is to examine whether the observed change in anion gap is matched by an equivalent change in serum bicarbonate level.
is to examine whether the observed change in anion gap is matched by an equivalent change in serum bicarbonate level.
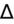
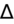 = (Calculated AG − Expected AG)/(24 − Measured HCO3−).
= (Calculated AG − Expected AG)/(24 − Measured HCO3−).
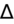
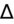 <1 = simultaneous non-AG acidosis.
<1 = simultaneous non-AG acidosis.
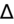
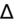 >2 = simultaneous metabolic alkalosis.
>2 = simultaneous metabolic alkalosis.
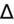
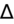 1–2 = pure AG metabolic acidosis.
1–2 = pure AG metabolic acidosis.
- Alternate method of measuring
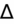
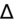 :
:
(Measured AG − Expected AG) + measured HCO3− = “New HCO3−”
“New HCO3−” >28 = simultaneous metabolic alkalosis, <20 = a simultaneous non–anion-gap acidosis.
- If an anion-gap acidosis is present, check urine and/or serum for possible etiologies: ketones, lactate, and metabolic panel to assess renal function for evidence of uremia (Table 44.2). If unrevealing, consider toxin screen and serum osmolality to check osmolal gap.
- Osmolal gap (OG) = measured osmoles − calculated osmoles (2 × Na+ + Glucose/18 + BUN/2.8 + EtOH/4.6).
- OG >10 suggests ingestion leading to unmeasured osmoles. The major ingestions that cause an elevated OG include various alcohols (ethanol, methanol, ethylene glycol, acetone, isopropyl alcohol), formaldehyde, paraldehyde, and diethyl ether. However, smaller ingestions can be missed and serum volatiles screen should also be checked.
- If a non–anion-gap acidosis is present and history is unrevealing, check urine anion gap = Urine Na+ + Urine K+ – Urine Cl−. Causes of non–anion gap acidosis are listed in Table 44.3.
- Urine anion gap is an indirect measurement of renal ammonium (NH4+) excretion, which is excreted with Cl−. The normal renal response to acidemia is to increase NH4+ excretion.
- A negative urine anion gap suggests increased renal NH4+ excretion consistent with diarrhea, type II renal tubular acidosis (RTA), or dilutional acidosis.
- A positive urine anion gap suggests impaired renal excretion of NH4+ consistent with type I or IV RTA or chronic kidney disease.
- A negative urine anion gap suggests increased renal NH4+ excretion consistent with diarrhea, type II renal tubular acidosis (RTA), or dilutional acidosis.
Table 44.2. Etiologies of anion-gap acidosis
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




