CHAPTER 10 Diagnosis of Acute Myocardial Infarction
MYOCARDIAL INFARCTION (MI) describes the process of myocardial cell death caused by ischemia, or the perfusion imbalance between supply and demand within the coronary arteries resulting from an acute thrombotic process. In the United States in 2006, approximately 16.8 million (7.6%) people had coronary heart disease, and an estimated 935,000 people experienced an acute MI that year, of which more than 150,000 resulted in death.1 In 2009, it was estimated that approximately every 25 seconds an American would have a coronary event, and about every minute an individual would die from one.1 The early recognition and diagnosis of acute MI is vital for the institution of therapy to limit myocardial damage and preserve cardiac function.
Acute coronary syndrome (ACS) refers to the constellation of clinical symptoms caused by active myocardial ischemia. Patients with ACS can be grouped into two major categories of acute MI: (1) patients with new ST segment elevation on the electrocardiogram (ECG) that is diagnostic of acute ST segment elevation myocardial infarction (STEMI), and (2) patients with non–ST segment elevation myocardial infarction (NSTEMI) who have positive cardiac biomarkers in an appropriate clinical setting, with or without ECG ST segment depression or T wave inversion.2
International registry data found that of patients who presented with an ACS, 25% experienced NSTEMI, whereas 30% had STEMI.3 Clinical trials have established the benefit of early reperfusion therapy in patients with STEMI and an early invasive strategy in patients with NSTEMI, and so a rapid and accurate assessment of patients with suspected acute MI is essential for optimal management.2,4 This chapter describes the diagnostic modalities for the evaluation of patients with suspected acute MI.
History
There have been considerable advances in the detection of myocardial injury and necrosis in the last several decades. As a result, the definition of MI has evolved over time. Beginning in the 1950s, the World Health Organization used epidemiologic data to define acute MI as the presence of at least two of the following three criteria: (1) clinical symptoms suggestive of myocardial ischemia, (2) ECG abnormalities, or (3) elevation in serum markers indicative of myocardial necrosis.5 The development of more sensitive and specific biomarkers of myocardial necrosis and precise imaging techniques for ischemic myocardial dysfunction has led to further refinement of the diagnosis of MI. In 2007, a Global Task Force assembled from the European Society of Cardiology, the American College of Cardiology, the American Heart Association, and the World Heart Federation published a consensus statement that sought to standardize cardiac biomarker detection, incorporate cardiac imaging into the evaluation of a patient with MI, and classify the different types of MIs, furthering the evolution of the definition of acute MI.6
Definition of Myocardial Infarction
MI is defined by the presence of myocardial necrosis combined with the clinical presentation of myocardial ischemia. The diagnosis of acute MI requires the increase or decrease (or both) of cardiac biomarkers (preferably troponin) with at least one value greater than the 99th percentile of the upper reference limit and at least one of the following: symptoms of ischemia, ECG changes indicative of active ischemia (new ST segment–T wave changes or new left bundle branch block [LBBB]), or imaging evidence of new regional wall motion abnormality or loss of viable myocardium.6 The type of MI can be classified further depending on the etiology of the infarct (Table 10-1).
Table 10–1 Classification of Myocardial Infarction (MI)
| Type | |
|---|---|
| 1 | Spontaneous MI resulting from a primary coronary event, such as coronary artery plaque erosion, or rupture, fissure, or dissection |
| 2 | MI associated with ischemia secondary to either increased oxygen demand or decreased supply, such as in coronary artery spasm, coronary embolism, anemia, arrhythmia, hypertension, or hypotension |
| 3 | Sudden unexpected cardiac death, including cardiac arrest, often with symptoms suggestive of myocardial ischemia, accompanied by new ST segment elevation, new left bundle branch block, or evidence of fresh thrombus in a coronary artery by angiography or at autopsy, but death occurring before blood samples could be obtained, or at a time before the appearance of cardiac biomarkers in the blood |
| 4a | MI associated with percutaneous coronary intervention |
| 4b | MI associated with stent thrombosis as documented by angiography or autopsy |
| 5 | MI associated with coronary artery bypass graft surgery |
Modified and adapted from Thygesen K, Alpert JS, White HD: Universal definition of myocardial infarction. J Am Coll Cardiol 2007;50:2173-2195.
Biochemical Markers of Acute Myocardial Infarction
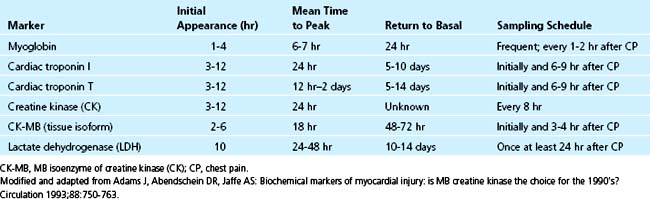
The ideal biochemical marker should be present in high concentration in the myocardium, absent in noncardiac tissue, released rapidly in a linear fashion after myocardial necrosis, and present in the serum long enough to be easily detectable by an inexpensive and widely available assay. Table 10-2 summarizes serum cardiac markers. Cardiac biomarkers are an essential component of the criteria used to establish the diagnosis of acute MI. Troponins have become the preferred biomarkers for the detection of myocardial necrosis and are a class I indication in the diagnosis of MI.6–8 The improved sensitivity and tissue specificity of cardiac troponins compared with creatine kinase MB (CK-MB) and other conventional cardiac biochemical markers of acute MI have been well established.8,9 Troponins are not only useful for diagnostic implications, but also impart prognostic information and can assist in the risk stratification of patients presenting with suspected ACS.
In addition to the established biomarkers of myocardial necrosis, B-type natriuretic peptide (BNP) and C-reactive protein (CRP) are pathologically diverse biomarkers that could potentially enhance risk stratification in ACS further. Finally, several novel markers of myocardial ischemia and their usefulness during acute MI are currently being evaluated in clinical studies. To date, measurement of more than one specific biomarker of myocardial necrosis is unnecessary for establishing the diagnosis of MI and is not currently recommended.10 Certain biomarkers should no longer be used in the evaluation of acute MI because of their poor specificity secondary to their wide tissue distribution, including aspartate aminotransferase, total lactate dehydrogenase, and lactate dehydrogenase isoenzymes.11
Detectable increases in cardiac biomarkers are indicative of myocardial injury. Biomarker elevations are not synonymous with acute MI, however. Many disease states, such as sepsis, hypovolemia, atrial fibrillation, congestive heart failure, pulmonary embolism, myocarditis, intracranial hemorrhage, stroke, and renal failure, can be associated with an increase in cardiac biomarkers. These elevations arise from mechanisms other than thrombotic coronary artery occlusion and require treatment of the underlying cause, rather than the administration of antithrombotic and antiplatelet agents.12,13
Troponin
Diagnosis
Troponin is released early in the course of acute MI. An increased concentration of cardiac troponin is defined as exceeding the 99th percentile of a reference control group. Troponin exceeding this limit on at least one occasion in the setting of clinical ischemia is indicative of myocardial necrosis.6 Elevated troponin can be detected within 2 to 4 hours after the onset of myocardial injury.13 Serum levels can remain increased for 4 to 7 days for troponin I and 10 to 14 days for troponin T (Fig. 10-1).14
The initial release of troponin is from the cellular cytosol, whereas the persistent elevation is a result of the slower dispersion of troponin from degrading cardiac myofilaments.15 As a result of these kinetics, the sensitivity of troponin increases with time. At 60 minutes after the onset of acute MI, the sensitivity is approximately 90%, but maximal sensitivity of troponin (approximately 99%) is not achieved until 6 or more hours after the initiation of myocardial necrosis.13 Blood samples for the measurement of troponin levels are recommended to be drawn at presentation and 6 to 9 hours later to optimize the clinical sensitivity for ruling in acute MI and the specificity for ruling out acute MI.8
The specificity of troponin I is approximately 85% to 95% with serial testing.16 As a result of this high tissue specificity, cardiac troponin is associated with fewer false-positive results in the setting of concomitant skeletal muscle injury than CK-MB. This inherent characteristic of troponin has been used in the diagnosis and assessment of myocardial injury in patients with chronic muscle diseases, in marathon runners, in patients after electrical cardioversion, in patients with cardiac contusions, and in patients with perioperative MIs.16–19 The tissue specificity of cardiac troponin is distinct from the specificity for the mechanism of myocardial injury; if elevated troponins are found in the absence of myocardial ischemia, an evaluation for alternative etiologies of myocardial injury should be pursued.
Despite the ongoing development of increasingly sensitive troponin assays, troponin kinetics do not reliably permit the very early (initial 1 to 3 hours) detection of myocardial necrosis.8 In patients presenting within 6 hours of symptom onset, the clinical scenario, ECG findings, and adjunctive imaging techniques are necessary for the rapid and accurate diagnosis of acute MI. In the case of STEMI, reperfusion therapy should not be delayed waiting for confirmatory biomarkers of myocardial injury.
Elevated troponins not only are vital to the diagnosis of NSTEMI, but also serve to direct treatment by identifying patients who would benefit from an early invasive management strategy.20 In the TACTICS-TIMI 18 study, patients with any increase in troponin who underwent early angiography (within 4 to 48 hours) and revascularization (if appropriate) achieved an approximately 55% reduction in the odds of death or MI compared with patients undergoing conservative management.21
Prognosis
In addition to the diagnostic value of troponin, cardiac troponins yield prognostic information. Prognosis is related partly to the extent of the increase in troponin in patients with an ischemic mechanism for myocardial injury.22–24 Increased concentrations of troponin are associated with angiographic findings of greater lesion complexity, impaired blood flow in the culprit artery, and decreased coronary microvascular perfusion.21
Cardiac troponin has also been proven to be a potent independent indicator of recurrent ischemic events and the risk of death among patients presenting with ACS.25 The TIMI-IIIB trial showed that in patients presenting with ACS, mortality was consistently higher among patients with elevated troponin I (>0.4 ng/mL) at the time of admission. There were statistically significant increases in mortality with increasing levels of troponin I. Even after adjustment for baseline variables, age older than 65, and ST segment depression on ECG, an elevated troponin I had the strongest impact on mortality.26 Additionally, the GUSTO IIa trial found that elevated troponin T (>0.1 ng/mL) was significantly predictive of 30-day mortality in patients with acute myocardial ischemia even after analysis was adjusted for ECG category and CK-MB level.27 In patients with STEMI, increased troponin is also associated with a significantly higher mortality at 30 days, which persisted even after adjustment for age, heart rate, systolic blood pressure, location of infarction, and Killip class.28
Risk Stratification
Cardiac troponin is a class I indication for risk stratification in patients with ACS.8 Patients presenting with clinical evidence of ischemia and positive troponins, even at low levels, have worse outcomes than patients without evidence of elevated troponin.29 The MISSION! trial showed that peak troponin T levels are a good estimate of infarct size and an independent predictor for left ventricular function at 3 months and major adverse cardiac events at 1 year.23
Creatine Kinase MB
CK is a cytosolic carrier protein for high-energy phosphates.13 CK-MB is an isoenzyme of CK that is most abundant in the heart; however, CK-MB also constitutes 1% to 3% of the CK in skeletal muscle, and is present in a small fraction in other organs, such as the small bowel, uterus, prostate, and diaphragm.30 The specificity of CK-MB may be impaired in the setting of major injury to these organs, especially skeletal muscle.
Although cardiac troponin is the preferred marker of myocardial necrosis, CK-MB by mass assay is an acceptable alternative when cardiac troponin is unavailable.8 The diagnostic limit for CK-MB is defined as the 99th percentile in a sex-specific reference control group.6 All assays for CK-MB show a significant twofold to threefold higher 99th percentile limit for men compared with women. In addition, CK-MB can have twofold to threefold higher concentrations in African Americans than whites. These discrepancies have been attributed to physiologic differences in muscle mass.11 It is recommended that two consecutive measurements of CK-MB above the diagnostic limit be required for sufficient evidence of myocardial necrosis because of the inherent lower tissue specificity of CK-MB compared with troponin.8
The temporal increase of CK-MB is similar to that of troponin in that it occurs within 3 to 4 hours after the onset of myocardial injury, but in contrast to troponin, CK-MB decreases to the normal range by 48 to 72 hours (see Fig. 10-1). The rapid decline of CK-MB to the reference interval by 48 to 72 hours allows for the discrimination of early reinfarction when symptoms recur between 72 hours and 2 weeks after the index acute MI, when troponin may still be elevated.8 More recent data suggest, however, that serial troponin I values provide similar information.31 Similar to troponin, the amount of CK-MB released is useful for estimation of infarct size, which correlates with ejection fraction, incidence of ventricular arrhythmias, and prognosis.14